Abstract
Although target of rapamycin (TOR) kinase and Ras are central regulators of cell growth in yeast and mammals, the molecular mechanisms underlying their regulation by nutrients are still poorly understood. Interestingly, recent studies identified cytosolic pH as a critical regulatory signal for both pathways, which might have widespread implications for tumor cell biology
Abbreviations
| C-source | = | Carbon source |
| mTORC1 | = | mammalian TOR complex 1 |
| NHE1 | = | Na+/H+ exchanger 1 |
| P-ATPase | = | Plasmamembrane ATPase |
| PKA | = | cAMP-dependent Protein Kinase A |
| TOR | = | Target Of Rapamycin |
| TORC1 | = | TOR Complex 1 |
| V-ATPase | = | Vacuolar ATPase |
Nutrients are a major cell growth determinant and regulate highly conserved signaling pathways to adjust cellular physiology to environmental conditions.Citation1 Although it is widely appreciated that metabolic function impacts health and disease, and multiple regulators of nutrient sensitive signaling pathways have been identified, little is known about the molecular mechanisms of nutrient sensing.Citation1,2
Importantly, nutrient sensing mechanisms need to integrate signals from structurally diverse nutrients, such as various sugars or amino acids. Thus, several sensors may exist that sense individual nutrients and redundantly activate downstream signaling pathways. Alternatively, a common metabolite might mediate sensing of different nutrients, triggering a single sensor to regulate cellular signaling. Although the latter model offers an elegant and intuitive explanation for this problem, and is also supported by available evidence, the metabolic signals regulating the key growth promoting pathways, including target of rapamycin complex 1 (TORC1) and cAMP-dependent protein kinase A (PKA), remain largely elusive.Citation1-3
Interestingly, several studies have recently identified cytosolic pH as a signal that regulates cell growth in response to different sugars in yeast.Citation4–6 Cytosolic pH is sensitive to the quality and quantity of the available carbon source (C-source), and correlates with growth rates under these conditions.Citation4,5 Genetic analysis revealed that high cytosolic pH is both sufficient and required to activate TORC1 and Ras activity upstream of PKA,Citation4 thereby readily explaining cell growth regulation through cytosolic pH ().
Figure 1. Cytosolic pH links glucose metabolism to the regulation of cell growth. In yeast, carbon source availability regulates cytosolic pH through modulation of plasma membrane ATPase (P-ATPase) activity. Cytosolic pH acts as a signal to trigger phosphorylation of Sch9 by target of rapamycin complex 1 (TORC1) and Ras activity upstream of cAMP-dependent protein kinase A (PKA) via vacuolar ATPase (V-ATPase). Note that V-ATPase interacts with different GTPases at different cellular compartments (golgi and vacuole) to regulate Ras and TORC1 activity. See text for details.
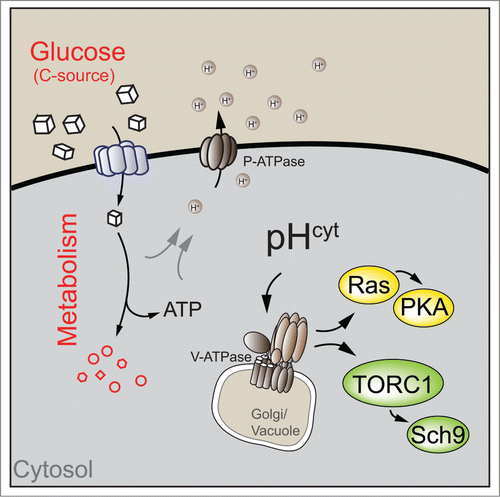
In yeast, cytosolic pH regulation is mostly mediated by plasma membrane ATPase (P-ATPase), an ATP-dependent proton pump located in the plasma membrane that links cellular metabolism to cytosolic pH regulation through a currently unknown mechanism. Since establishing high cytosolic pH consumes a large fraction of cellular ATP,Citation1 it seems plausible that P-ATPase activity is tightly linked to the energy status (e.g., the ATP/ADP ratio) of the cell. Alternatively, direct coupling of P-ATPase activity to glycolytic flux might offer an attractive hypothesis for this regulation, yet evidence for flux sensing mechanisms remains largely circumstantial.Citation7
Nevertheless, cytosolic pH possesses some unique features that make it ideally suited to act as a signal regulating cell growth. As C-sources fuel central carbon metabolism to produce ATP and cellular building blocks with different efficiencies, the resulting differences in cytosolic pH may directly link growth to cellular metabolism and explain how growth is regulated by these signals. In addition, cytosolic pH can also easily integrate other environmental signals and stresses via multiple mechanisms. For example, our unpublished data demonstrate that oxidative stress induced by addition of H2O2 rapidly reduces cytosolic pH, a response that might contribute to cellular adaptation and growth arrest.
We have previously demonstrated that cytosolic pH is sensed by vacuolar ATPase (V-ATPase), a proton pump required for intraluminal acidification of the endomembrane system, most notably the vacuole. High cytosolic pH promotes assembly and activation of V-ATPase,Citation6 which is required for full Ras and TORC1 activity.Citation4 Interestingly, V-ATPase activates TORC1 and Ras activity by recruitment and activation of distinct small GTPases, which link V-ATPase to downstream signaling cascades. Specifically, V-ATPase activates Arf1 and its partially redundant homolog Arf2 to trigger Ras activity. While the mechanism of Ras activation remains to be established, Arf1 might promote Ras localization at the plasma membrane and thus enhance its interaction with activators and downstream targets.
Similarly, genetic and biochemical evidence suggests that V-ATPase also interacts with Gtr1 and Gtr2,Citation4 the yeast homologues of Rag GTPases, which activate TORC1 in response to amino acids in yeast and mammals.Citation2,3 These data suggest a model in which glucose and amino acids converge on a single activator to trigger TORC1 activity. As C-source availability is required for V-ATPase, and consequently for Gtr1 and Gtr2 activity, the presence of a C-source is a prerequisite for TORC1 activation by amino acids. Similarly, recent evidence demonstrates that glucose and amino acid signaling in mammalian cells also converges on Rag GTPases to promote mTORC1 activity, possibly in a V-ATPase dependent manner.Citation2,8 Although direct evidence for the regulation of mTORC1 or Ras activity by cytosolic pH in mammalian cells is lacking, it is tempting to speculate that cytosolic pH might be a conserved cellular signal mediating nutrient sensing. Indeed, available evidence suggests that cytosolic pH might also promote cell growth in this system. Most notably, increased cytosolic pH has been associated with cellular transformation and is considered one of the hallmarks of cancer cells.Citation9
Regulation of cytosolic pH in mammalian cells mostly relies on the Na+/H+ exchanger 1 (NHE1). Interestingly, NHE1 is phosphorylated in a growth factor-dependent manner and functional studies support a role of NHE1 activation and increased cytosolic pH in cell growth regulation.Citation9 For example, pharmacological inhibition of NHE1 by amiloride blocks an increase in cytosolic pH and cell cycle progression triggered by injection of a dominant active Ras protein into arrested cells.Citation10
Nonetheless, how cytosolic pH might promote mammalian cell growth is unclear. Increased cytosolic pH might simply thermodynamically favor certain biochemical reactions supporting cell growth, for example by enhancing glycolytic flux.Citation9 Similarly, altered regulation of cytosolic pH could also indirectly affect the growth of cancer cells by increased extracellular acidification to generate a microenvironment that is more favorable for growth. However, our yeast data demonstrating that cytosolic pH regulates growth by modulating TORC1 and Ras activity offer the exciting possibility that cytosolic pH has evolved as a conserved, specific signal that regulates nutrient sensitive signaling pathways to promote growth. Therefore, further studies directed to understanding the potential signaling function of cytosolic pH in mammalian cells should not only lead to a better understanding of cellular physiology in normal and cancer cells, but might also open new possibilities for therapeutic interventions for this disease.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Shady Saad and Alfredo Ibáñez for helpful discussions and Alicia Smith for comments on the manuscript.
Funding
This work was supported by the European Research Council (ERC), the Swiss National Science Foundation (SNF) and the ETH Zürich.
References
- Saad S, Peter M, Dechant R. In scarcity and abundance: metabolic signals regulating cell growth. Physiology (Bethesda) 2013; 28:298-309; PMID:23997189; http://dx.doi.org/10.1152/physiol.00005.2013
- Bar-Peled L, Sabatini DM. Regulation of mTORC1 by amino acids. Trends Cell Biol 2014; 24:400-6; PMID:24698685; http://dx.doi.org/10.1016/j.tcb.2014.03.003
- Broach JR. Nutritional control of growth and development in yeast. Genetics 2012; 192:73-105; PMID:22964838; http://dx.doi.org/10.1534/genetics.111.135731
- Dechant R, Saad S, Ibanez AJ, Peter M. Cytosolic pH Regulates Cell Growth through Distinct GTPases, Arf1 and Gtr1, to Promote Ras/PKA and TORC1 Activity. Mol Cell 2014; 55:409-21; PMID:25002144; http://dx.doi.org/10.1016/j.molcel.2014.06.002
- Orij R, Urbanus ML, Vizeacoumar FJ, Giaever G, Boone C, Nislow C, Brul S, Smits GJ. Genome-wide analysis of intracellular pH reveals quantitative control of cell division rate by pHc in Saccharomyces cerevisiae. Genome biology 2012; 13:R80; PMID:23021432; http://dx.doi.org/10.1186/gb-2012-13-9-r80
- Dechant R, Binda M, Lee SS, Pelet S, Winderickx J, Peter M. Cytosolic pH is a second messenger for glucose and regulates the PKA pathway through V-ATPase. EMBO J 2010; 29:2515-26; PMID:20581803; http://dx.doi.org/10.1038/emboj.2010.138
- Kochanowski K, Volkmer B, Gerosa L, Haverkorn van Rijsewijk BR, Schmidt A, Heinemann M. Functioning of a metabolic flux sensor in Escherichia coli. Proc Natl Acad Sci U S A 2013; 110:1130-5; PMID:23277571; http://dx.doi.org/10.1073/pnas.1202582110
- Efeyan A, Zoncu R, Chang S, Gumper I, Snitkin H, Wolfson RL, Kirak O, Sabatini DD, Sabatini DM. Regulation of mTORC1 by the Rag GTPases is necessary for neonatal autophagy and survival. Nature 2013; 493:679-83; PMID:23263183; http://dx.doi.org/10.1038/nature11745
- Harguindey S, Arranz JL, Polo Orozco JD, Rauch C, Fais S, Cardone RA, et al. Cariporide and other new and powerful NHE1 inhibitors as potentially selective anticancer drugs–an integral molecular/biochemical/metabolic/clinical approach after one hundred years of cancer research. Journal of translational medicine 2013; 11:282; PMID:24195657; http://dx.doi.org/10.1186/1479-5876-11-282
- Hagag N, Lacal JC, Graber M, Aaronson S, Viola MV. Microinjection of ras p21 induces a rapid rise in intracellular pH. Mol Cell Biol 1987; 7:1984-8; PMID:3037340
