Abstract
Galectin-12, a member of the galectin family of animal lectins, is preferentially expressed in adipocytes. We recently reported that this galectin is localized on lipid droplets, specialized organelles for fat storage. Galectin-12 regulates lipid degradation (lipolysis) by modulating lipolytic protein kinase A (PKA) signaling. Mice deficient in galectin-12 exhibit enhanced adipocyte lipolysis, increased mitochondria respiration, reduced adiposity and ameliorated insulin resistance associated with weight gain. The results suggest that galectin-12 may be a useful target for treatment of obesity-related metabolic conditions, such as insulin resistance, metabolic syndrome, and type 2 diabetes. Most previously described galectins largely reside in the cytosol, although they can also be induced to become associated with membrane-containing structures. Along with an in-depth characterization of galectin-12, this mini-review comments on this first report of a galectin normally localized specifically in an organelle that performs an important intracellular function. Further studies will help shed light on how this protein regulates cellular homeostasis, especially energy homeostasis, and provide additional insight into the intracellular functions of galectins.
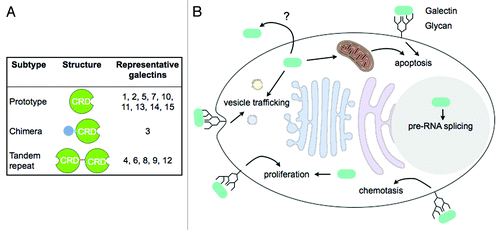
Adipocytes, cells that are the primary cellular component of adipose tissue, play a crucial role in energy homeostasis. They are specialized to store fatty acids as triglycerides and release free fatty acids and glycerol according to an organism’s energy status. Energy intake in excess of energy expenditure, often as a result of overeating, can lead to lipid overload in adipocytes that causes obesity and predisposes the animal to other metabolic disorders such as insulin resistance and type 2 diabetes.Citation1 The protein in question in our recent paper published in the Proceedings of the National Academy of Sciences of the United States of America,Citation2 galectin-12, is a member of the galectin family that includes 15 animal lectins so far identified containing one or two carbohydrate-recognition domains (CRDs) with conserved consensus sequences and affinity for β-galactosides (). The members exhibit considerable diversity in binding preference that depends on the presentation of β-galactosides in the glycans.Citation3 Unlike many other animal lectins that mainly function from outside the cells, galectins reside intracellularly, although they can be secreted, and can function from both inside and outside the cell in diverse cellular processes ( and reviewed in refs. Citation3–Citation5).
Figure 1. Galectin structure and function. (A) Galectin subtypes and structural features. Prototypical galectins are composed of a single galectin CRD. The chimeric type of galectin, galectin-3, consists of a N-terminal non-lectin domain in addition to a C-terminal galectin CRD. Tandem repeat type of galectins contains 2 CRDs in tandem connected by a linker sequence. (B) Galectins are synthesized in the cytosol but can also be secreted through poorly-defined mechanisms. Functioning from both outside and inside of the cell, galectins are involved in diverse cellular processes including vesicle trafficking of glycoproteins, chemotaxis, proliferation, pre-RNA splicing, and apoptosis. Extracellular galectins are likely to function by binding to cell surface glycans, while intracellular effectors that mediate galectin function are largely unknown.
Galectin-12, a two-CRD galectin, was cloned a decade ago by this laboratory.Citation6 Like other galectin genes, galectin-12 gene (Lgals12) does not encode a signal peptide, suggesting that it codes for an intracellular protein. The predicted protein exhibits considerable divergence from consensus sequence among the galectin family of proteins. Whereas the percentage of sequence identity among most human galectin CRDs ranges from 20% to 50%, the C-terminal CRD of galectin-12 is less than 20% identical to most other galectin CRDs and lack several amino acid residues that are known to be essential for carbohydrate binding. The gene was found to be predominately expressed by adipose tissue.Citation7 The protein was previously shown to arrest the cell cycle at G1 phase when ectopically expressed in some cancer cellsCitation6 and to induce apoptosis in others.Citation7 Kouadjo et al. further reported that in mice, Lep (encoding the adipocyte hormone leptin) and Lgals12 were the only two genes exclusively expressed in adipose tissue.Citation8 The remarkable tissue specificity in galectin-12 expression suggested that this protein might play an important role in the biology of adipocytes. Indeed, we have now reported that galectin-12 suppresses lipolysis in adipocytes. Ablation of this protein in mice leads to increased lipolysis, decreased adiposity and amelioration of insulin resistance associated with weight gain.Citation2
Galectin-12 Regulates Lipolysis in Lipid Droplets
Most galectins are cytosolic proteins, although some can be induced to become associated with membrane structures, such as lipid rafts, immunological synapses, phagosomes and mitochondria.Citation3 It is therefore a surprise that galectin-12 is not detectable in the cytosol, but is mainly associated with lipid droplets under normal conditions.Citation2 Lipid droplets consist of a phospholipid monolayer that surrounds a core of neutral lipids, such as sterol esters and triglycerides, with a surface that is decorated with a number of proteins, many of which play functional roles in lipid droplet biology.Citation9 Although being ubiquitous organelles found in most eukaryotic cells, they are especially prominent in adipocytes. Lipid droplets are the major sites of energy storage and catabolism, processes highly regulated by hormones and metabolic signaling pathways.Citation10
Like mRNA for other galectins, galectin-12 mRNA lacks sequences that encode a classical signal peptide.Citation6 Thus, the protein is likely to be synthesized in free ribosomes and then directed to the preexisting lipid droplets. This is similar to the PERILIPIN family of proteinsCitation11,Citation12 and distinct from other non-residential lipid droplet proteins, such as caveolin and DGAT2, which are synthesized in the ER and transferred from the endoplasmic leaflet of the ER membrane to the lipid droplets by inundating lipids during lipid droplet formation or growth (reviewed in ref. Citation9). The lack of association of galectin-12 with other cellular membranes suggests that the monolayer membrane surface of lipid droplets is somewhat unique. How galectin-12 distinguishes between the mono-layer surface of lipid droplets and the bilayer surfaces of other organelles is not known. One possibility is there is a dynamic interplay between galectin-12 and lipid droplet-specific lipids or proteins. The detailed mechanism of how galectin-12 is localized to lipid droplet is unknown but is likely to involve hydrophobic regions scattered throughout the galectin-12 protein sequence.Citation2
Since the discovery of the regulation of lipolysis by the lipid droplet protein PERILIPIN 1 (PLIN1),Citation13-Citation15 it is clear that the metabolic function of these organelles is in part mediated by their associated proteins. PLIN1 acts both as a barrier that limits the access of lipases to the core of triglycerides and as a regulatory protein that controls lipase activation and translocation.Citation16 Ablation of PLIN1 exposes the lipid droplet core to the actions of lipases and results in maximal lipolysis under basal conditions. Incubation with catecholamines does not produce further stimulation of lipolysis in PLIN1-deficient adipocyte.Citation14,Citation15 In contrast, ablation of galectin-12 leads to enhancement of both basal and catecholamine-stimulated lipolysis.Citation2 The results suggest that unlike PLIN1, galectin-12 mainly acts as a regulator of pro-lipolytic signaling and not as a major structural protein that shields the lipid droplet core from the actions of lipases. This is consistent with the finding that galectin-12 is preferentially localized to certain surface domains of large lipid droplets, which are known to be less sensitive to lipolytic stimulation than those smaller droplets.Citation17
Protein kinase A (PKA) signaling is a major regulator of lipolysis. PKA signaling is known to be compartmentalized signal emanated by a specific stimulus is directed to particular cellular sites where a functionally-related subset of PKA substrates are phosphorylated and activated (or inactivated).Citation18,Citation19 Galectin-12 may be localized to such domains of lipolytic PKA signaling and contribute to the compartmentalization of PKA signaling by acting as a scaffold protein that interacts with specific signaling molecules, as proposed by Baum.Citation20 This is analogous to another galectin, galectin-3, which has been shown to function as a scaffold protein in the formation of nanoclusters with K-ras on the plasma membrane.Citation21 It thus can be predicted that galectin-12 ablation preferentially affects PKA signaling that targets lipolysis, while other PKA-activated cellular processes, such as activation of CREB-mediated transcription in the nucleus, are not affected. This remains to be confirmed experimentally.
Galectin-12-deficient adipocytes also exhibit enhanced mitochondrial respiration.Citation2 Although this can be explained by increased availability of fuels (fatty acids) to mitochondria as a result of enhanced lipolysis in these cells, a more direct role for galectin-12 in mitochondrial activity cannot be excluded. Some mitochondria are located in close association with lipid droplets in adipocytes and there could be an exchange of surface materials between the two organelles. It will be interesting to determine whether galectin-12 exists in this subset of mitochondria. Galectin-12 at the interface of the two organelles could also regulate local lipid fluxes of fatty acids from the lipid droplets to mitochondria for oxidation. In any rate, increased fatty acid oxidation and mitochondrial respiration in the face of enhanced lipolysis may contribute to greater energy expenditure in Lgals12−/− animals. A combination of these factors reduces adiposity and in the meantime prevents a rise of fatty acid levels in the circulation and limits lipid deposition in insulin sensitive tissues, resulting in improved insulin sensitivity.Citation2 It should be noted, however, that normal amount of adipose tissue is essential for the maintenance of energy homeostasis,Citation22-Citation25 and genetic manipulations that reduce the capacity of fat storage in this tissue often lead to increased fatty acid levels in the circulation, ectopic deposition of lipids in muscle and liver, and impaired insulin sensitivity.Citation15,Citation26-Citation28
Roles of Galectin-12 in Other Cells
While galectin-12 is predominately produced in adipocytes in mice, it is also expressed in human leukocytes, especially myeloid cells.Citation6 Species differences in the temporal and spatial expression of some genes are not uncommon. For example, the adipokine resistin that exacerbates adipose tissue inflammation and insulin resistance is produced exclusively by adipose tissue in mice, but mainly expressed in macrophages in humans.Citation29,Citation30 Major adipogenic transcription factors such as the C/EBP and PPAR families of transcription factors also play critical roles in myeloid differentiation and function.Citation31-Citation34 Thus, adipocytes and cells of myeloid origin may be much more similar than previously considered. Indeed, it has recently been reported that white adipocytes can be generated from the myeloid lineage via mesenchymal intermediates.Citation35
Leukocytes actively accumulate lipid droplets in infectious and other inflammatory conditions.Citation36,Citation37 In addition to its well-known role as a site for lipid storage, lipid droplets also house a variety of signaling molecules that regulate not only energy metabolism, but also many other cellular processes. A key role for this organelle as an energy store and a cytoplasmic domain that organizes intracellular signaling in immune responses is coming to light.Citation37,Citation38 Whether galectin-12 is also localized to lipid droplets in leukocytes and its precise role in the immune response remain to be determined.
Roles of the Carbohydrate-Recognition Domains in Intracellular Galectin-12 Function
While galectins are able to exert various activities in vitro by engaging cell surface glycans, as demonstrated by addition of recombinant proteins to cell lines or primary cells from humans or animals, it has become increasingly clear they can regulate cellular responses by functioning intracellularly.Citation3 Our findings of the role of galectin-12 in regulation of lipolysis and its localization to lipid droplets provide another example of intracellular functions.
At this time, most studies of galectins have considered the carbohydrate-binding regions as the functional sites that elicit direct cellular responses through binding to glycans. However, it is also possible for these regions to serve as regulatory sites whose occupancy regulates (instead of mediates) galectin function. It has been shown that carbohydrate binding to the C-terminal CRD of galectin-3 affects its N-terminal domain-mediated self-association.Citation39 Although such regulatory molecules for galectin-12 remain to be identified, they are likely to be intracellular metabolic intermediates that may or may not be carbohydrates in structure. Binding of these molecules to the presumably carbohydrate-binding sites of galectin-12 may affect its interaction with as yet unknown effector molecules that mediate its metabolic function. A major theme in metabolic regulation is that alterations of nutritional status result in fluctuations in the levels of metabolic intermediates, which in turn allosterically regulate enzymes of their respective metabolic pathways to achieve metabolic homeostasis. We now propose that the metabolic function of galectin-12 may be similarly regulated to help maintain energy homeostasis. It is to be noted also that galectin-12 CRDs, especially the C-terminal one, display considerable divergence from other galectin CRDs.Citation6 Although the protein retains some lactose-binding activity (likely contributed by its N-terminal CRD),Citation6,Citation7 it is possible that the C-terminal domain, which lacks several of the key amino acid residues known to be crucial for β-galactoside binding, has evolved to preferentially bind ligands other than carbohydrates.
Concluding Remarks
An important role for galectin-12 in the regulation of lipolysis and whole-body energy metabolism has thus been identified (). Yet many crucial questions remain to be answered. What are the functions of galectin-12 in other cell types, especially in humans where its expression is not as restricted to adipocytes as it is in mice? Are different galectin-12 functions mediated by distinct or same factors? What is the relevance of the carbohydrate-binding sites for such an intracellular lectin? How are the different functions of galectin-12 regulated? These and other unanswered questions remind us that we are just beginning to unravel the many mysteries of this intriguing protein. Further studies of localization and identification of cellular components that interact with galectin-12 will help shed light on how this protein regulates cellular homeostasis, especially energy homeostasis, as well as providing additional insight into the intriguing family of galectins. Lastly, galectin-12 may be a useful target for the management of obesity-related metabolic conditions, such as insulin resistance, metabolic syndrome and type-2 diabetes.
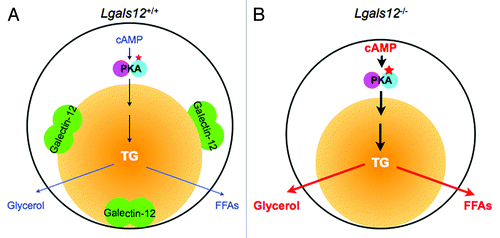
Figure 2. Role of galectin-12 in the regulation of lipolysis in adipocytes by protein kinase A (PKA) signaling. (A) Lipolysis in a wildtype (Lgals12+/+) adipocyte. PKA is activated by cyclic AMP (cAMP) to set off a signaling cascade that results in the sequential hydrolysis of triglyceride (TG) in the lipid droplet (yellow ball) to free fatty acids (FFAs) and glycerol. In this cell, the lipid droplet protein galectin-12 limits cAMP levels to downregulate PKA activation, most likely by acting on specific phosphodiesterases that degrade cAMP. (B) Ablation of galectin-12 in adipocytes results in elevated concentrations of cAMP, enhanced lipolytic PKA signaling, and therefore augmentation of lipolysis.
References
- Havel PJ. Update on adipocyte hormones: regulation of energy balance and carbohydrate/lipid metabolism. Diabetes 2004; 53:Suppl 1 S143 - 51; http://dx.doi.org/10.2337/diabetes.53.2007.S143; PMID: 14749280
- Yang RY, Yu L, Graham JL, Hsu DK, Lloyd KC, Havel PJ, et al. Ablation of a galectin preferentially expressed in adipocytes increases lipolysis, reduces adiposity, and improves insulin sensitivity in mice. Proc Natl Acad Sci U S A 2011; 108:18696 - 701; http://dx.doi.org/10.1073/pnas.1109065108; PMID: 21969596
- Yang RY, Rabinovich GA, Liu FT. Galectins: structure, function and therapeutic potential. Expert Rev Mol Med 2008; 10:e17; http://dx.doi.org/10.1017/S1462399408000719; PMID: 18549522
- Liu FT, Patterson RJ, Wang JL. Intracellular functions of galectins. Biochim Biophys Acta 2002; 1572:263 - 73; http://dx.doi.org/10.1016/S0304-4165(02)00313-6; PMID: 12223274
- Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer 2005; 5:29 - 41; http://dx.doi.org/10.1038/nrc1527; PMID: 15630413
- Yang RY, Hsu DK, Yu L, Ni J, Liu FT. Cell cycle regulation by galectin-12, a new member of the galectin superfamily. J Biol Chem 2001; 276:20252 - 60; http://dx.doi.org/10.1074/jbc.M010914200; PMID: 11283015
- Hotta K, Funahashi T, Matsukawa Y, Takahashi M, Nishizawa H, Kishida K, et al. Galectin-12, an Adipose-expressed Galectin-like Molecule Possessing Apoptosis-inducing Activity. J Biol Chem 2001; 276:34089 - 97; http://dx.doi.org/10.1074/jbc.M105097200; PMID: 11435439
- Kouadjo KE, Nishida Y, Cadrin-Girard JF, Yoshioka M, St-Amand J. Housekeeping and tissue-specific genes in mouse tissues. BMC Genomics 2007; 8:127; http://dx.doi.org/10.1186/1471-2164-8-127; PMID: 17519037
- Farese RVJ Jr., Walther TC. Lipid droplets finally get a little R-E-S-P-E-C-T. Cell 2009; 139:855 - 60; http://dx.doi.org/10.1016/j.cell.2009.11.005; PMID: 19945371
- Duncan RE, Ahmadian M, Jaworski K, Sarkadi-Nagy E, Sul HS. Regulation of lipolysis in adipocytes. Annu Rev Nutr 2007; 27:79 - 101; http://dx.doi.org/10.1146/annurev.nutr.27.061406.093734; PMID: 17313320
- Greenberg AS, Egan JJ, Wek SA, Garty NB, Blanchette-Mackie EJ, Londos C. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J Biol Chem 1991; 266:11341 - 6; PMID: 2040638
- Brasaemle DL. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res 2007; 48:2547 - 59; http://dx.doi.org/10.1194/jlr.R700014-JLR200; PMID: 17878492
- Kimmel AR, Brasaemle DL, McAndrews-Hill M, Sztalryd C, Londos C. Adoption of PERILIPIN as a unifying nomenclature for the mammalian PAT-family of intracellular lipid storage droplet proteins. J Lipid Res 2010; 51:468 - 71; http://dx.doi.org/10.1194/jlr.R000034; PMID: 19638644
- Martinez-Botas J, Anderson JB, Tessier D, Lapillonne A, Chang BH, Quast MJ, et al. Absence of perilipin results in leanness and reverses obesity in Lepr(db/db) mice. Nat Genet 2000; 26:474 - 9; http://dx.doi.org/10.1038/82630; PMID: 11101849
- Tansey JT, Sztalryd C, Gruia-Gray J, Roush DL, Zee JV, Gavrilova O, et al. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc Natl Acad Sci U S A 2001; 98:6494 - 9; http://dx.doi.org/10.1073/pnas.101042998; PMID: 11371650
- Brasaemle DL, Subramanian V, Garcia A, Marcinkiewicz A, Rothenberg A. Perilipin A and the control of triacylglycerol metabolism. Mol Cell Biochem 2009; 326:15 - 21; http://dx.doi.org/10.1007/s11010-008-9998-8; PMID: 19116774
- Moore HP, Silver RB, Mottillo EP, Bernlohr DA, Granneman JG. Perilipin targets a novel pool of lipid droplets for lipolytic attack by hormone-sensitive lipase. J Biol Chem 2005; 280:43109 - 20; http://dx.doi.org/10.1074/jbc.M506336200; PMID: 16243839
- Zhang J, Hupfeld CJ, Taylor SS, Olefsky JM, Tsien RY. Insulin disrupts beta-adrenergic signalling to protein kinase A in adipocytes. Nature 2005; 437:569 - 73; http://dx.doi.org/10.1038/nature04140; PMID: 16177793
- Jarnaess E, Taskén K. Spatiotemporal control of cAMP signalling processes by anchored signalling complexes. Biochem Soc Trans 2007; 35:931 - 7; http://dx.doi.org/10.1042/BST0350931; PMID: 17956249
- Baum LG. Burn control, an adipocyte-specific function for galectin-12. Proc Natl Acad Sci U S A 2011; 108:18575 - 6; http://dx.doi.org/10.1073/pnas.1115738108; PMID: 22058227
- Shalom-Feuerstein R, Plowman SJ, Rotblat B, Ariotti N, Tian T, Hancock JF, et al. K-ras nanoclustering is subverted by overexpression of the scaffold protein galectin-3. Cancer Res 2008; 68:6608 - 16; http://dx.doi.org/10.1158/0008-5472.CAN-08-1117; PMID: 18701484
- Gavrilova O, Marcus-Samuels B, Graham D, Kim JK, Shulman GI, Castle AL, et al. Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. J Clin Invest 2000; 105:271 - 8; http://dx.doi.org/10.1172/JCI7901; PMID: 10675352
- Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol 2008; 9:367 - 77; http://dx.doi.org/10.1038/nrm2391; PMID: 18401346
- Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev 2002; 23:201 - 29; http://dx.doi.org/10.1210/er.23.2.201; PMID: 11943743
- Trayhurn P, Beattie JH. Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc Nutr Soc 2001; 60:329 - 39; http://dx.doi.org/10.1079/PNS200194; PMID: 11681807
- Jaworski K, Ahmadian M, Duncan RE, Sarkadi-Nagy E, Varady KA, Hellerstein MK, et al. AdPLA ablation increases lipolysis and prevents obesity induced by high-fat feeding or leptin deficiency. Nat Med 2009; 15:159 - 68; http://dx.doi.org/10.1038/nm.1904; PMID: 19136964
- Shimomura I, Hammer RE, Richardson JA, Ikemoto S, Bashmakov Y, Goldstein JL, et al. Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adipose tissue: model for congenital generalized lipodystrophy. Genes Dev 1998; 12:3182 - 94; http://dx.doi.org/10.1101/gad.12.20.3182; PMID: 9784493
- Moitra J, Mason MM, Olive M, Krylov D, Gavrilova O, Marcus-Samuels B, et al. Life without white fat: a transgenic mouse. Genes Dev 1998; 12:3168 - 81; http://dx.doi.org/10.1101/gad.12.20.3168; PMID: 9784492
- Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, et al. The hormone resistin links obesity to diabetes. Nature 2001; 409:307 - 12; http://dx.doi.org/10.1038/35053000; PMID: 11201732
- Patel L, Buckels AC, Kinghorn IJ, Murdock PR, Holbrook JD, Plumpton C, et al. Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem Biophys Res Commun 2003; 300:472 - 6; http://dx.doi.org/10.1016/S0006-291X(02)02841-3; PMID: 12504108
- Yamanaka R, Lekstrom-Himes J, Barlow C, Wynshaw-Boris A, Xanthopoulos KG. CCAAT/enhancer binding proteins are critical components of the transcriptional regulation of hematopoiesis (Review). Int J Mol Med 1998; 1:213 - 21; PMID: 9852222
- Reddy VA, Iwama A, Iotzova G, Schulz M, Elsasser A, Vangala RK, et al. Granulocyte inducer C/EBPalpha inactivates the myeloid master regulator PU.1: possible role in lineage commitment decisions. Blood 2002; 100:483 - 90; http://dx.doi.org/10.1182/blood.V100.2.483; PMID: 12091339
- Moore KJ, Rosen ED, Fitzgerald ML, Randow F, Andersson LP, Altshuler D, et al. The role of PPAR-gamma in macrophage differentiation and cholesterol uptake. Nat Med 2001; 7:41 - 7; http://dx.doi.org/10.1038/83328; PMID: 11135614
- Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature 2007; 447:1116 - 20; http://dx.doi.org/10.1038/nature05894; PMID: 17515919
- Majka SM, Fox KE, Psilas JC, Helm KM, Childs CR, Acosta AS, et al. De novo generation of white adipocytes from the myeloid lineage via mesenchymal intermediates is age, adipose depot, and gender specific. Proc Natl Acad Sci U S A 2010; 107:14781 - 6; http://dx.doi.org/10.1073/pnas.1003512107; PMID: 20679227
- Melo RC, D’Avila H, Wan HC, Bozza PT, Dvorak AM, Weller PF. Lipid bodies in inflammatory cells: structure, function, and current imaging techniques. J Histochem Cytochem 2011; 59:540 - 56; http://dx.doi.org/10.1369/0022155411404073; PMID: 21430261
- Bozza PT, Viola JP. Lipid droplets in inflammation and cancer. Prostaglandins Leukot Essent Fatty Acids 2010; 82:243 - 50; http://dx.doi.org/10.1016/j.plefa.2010.02.005; PMID: 20206487
- Bougnères L, Helft J, Tiwari S, Vargas P, Chang BH, Chan L, et al. A role for lipid bodies in the cross-presentation of phagocytosed antigens by MHC class I in dendritic cells. Immunity 2009; 31:232 - 44; http://dx.doi.org/10.1016/j.immuni.2009.06.022; PMID: 19699172
- Hsu DK, Zuberi RI, Liu FT. Biochemical and biophysical characterization of human recombinant IgE-binding protein, an S-type animal lectin. J Biol Chem 1992; 267:14167 - 74; PMID: 1629216