Abstract
There are many unanswered questions related to the heterogeneity of adipose tissue depots and the paucity of their function, development, and organization at the cellular level. Much effort has been directed at studying white adipose tissue (WAT), the driver of obesity and the associated metabolic disease. In recent years, the importance of brown adipose tissue (BAT) has also been appreciated. While BAT depots are prominent in many small mammal species, their detection in adult humans has been technically challenging and the identity of brown human adipocytes found within depots of WAT has remained controversial. We recently reported a peptide probe that binds to BAT vasculature and, when coupled with a near-infrared fluorophore, can be used to detect BAT in whole body imaging. This probe reliably discriminates between endothelium associated with brown or brown-like (beige/brite) adipocytes and endothelium of visceral WAT. Improved probes based on this approach could aid in assessing human adipose tissue body distribution and remodeling, which is a process underlying various pathologies. This commentary aims at discussing open questions that need to be addressed before full clinical advantage can be taken from adipose tissue imaging, as well as its metabolic activation strategies.
Overview of Adipose Tissue Types
Mammalian body contains two main types of fat: white adipose tissue (WAT) and brown adipose tissue (BAT). Normal physiological function of WAT is to store excess energy as neutral triglycerides in the differentiated cells of WAT (white adipocytes), from which it can be rapidly released as needed.Citation1 Overgrowth of WAT is responsible for obesity, a medical condition affecting all ages and socioeconomic groups, which is causing escalating social concern.Citation2 Over the past decade, profound changes in nutrition and lifestyle in developed countries have led to a sharp increase in the prevalence of obesity. One of the key obesity complications is the metabolic syndrome, featured by systemic inflammation, insulin resistance, and dyslipidemia, which are associated with type 2 diabetes, cardiovascular disease, and some types of cancer.Citation3,Citation4
As opposed to WAT, BAT is responsible for thermogenic energy dissipation.Citation5,Citation6 Heat generation by brown adipocytes relies on their numerous mitochondria responsible for the tissue color and requires uncoupling protein 1 (UCP1) that leaks proton to uncouple substrate oxidation from ATP synthesis allowing for fast substrate oxidation with a low rate of ATP production. Upon uptake by brown adipocytes, a fraction of calories become dissipated as heat instead of being stored contributing to cell hypertrophy.Citation5 The function of BAT has been established predominantly through studies in rodents that contain substantial BAT depots through the adulthood. In humans, BAT is clearly present and active in newborns, whereas adults had been previously thought to lack BAT. Recently, the presence of functional BAT-like tissue has been demonstrated in adults.Citation7-Citation10 A number of results from the rodent models indicate that BAT has a protective effect against the pathological consequences of obesity.Citation11,Citation12 Therefore, the significance of adult BAT discovery lies in possible new avenues in treatment of obesity and the associated disorders.Citation13
BAT Imaging Challenges
The presence of BAT in adult humans has been demonstrated in adults through the use of positron emission tomography (PET) based on the uptake of fluorine-18 deoxyglucose (FDG) by metabolically active BAT combined with CT (CT).Citation7-Citation10 Since the original report of adult human BAT,Citation14 hypermetabolic adipose depots have been clearly identified in the cervical, supraclavicular, paravertebral, mediastinal, para-aortic, and suprarenal regions, while the presence of adult interscapular BAT has been debated.Citation14,Citation15 In both males and female patients pre-subjected to cold temperature, residual BAT dispersed in WAT and muscle are often revealed by PET, with likelihood of detection decreasing with age.Citation7,Citation9 Obesity has been identified as one of the factors that decrease the likelihood of detecting BAT based on FDG uptake.Citation10 An important conclusion made in this latter study is that both lean and obese adults have BAT “patches” confirmed by biopsy, although it is often metabolically inactive in obese individuals and is therefore undetectable by PET. Comprehensive quantification of the incidence and distribution of brown adipocytes has not been so far been possible due to the lack of appropriate methodology.
At present, the fundamental step to be taken in the field is the establishment of approaches to reliable localization and quantification of BAT depots. Methods to detection of BAT based on assays not depending on its metabolic activity would have significant advantages to PET. One would not need to subject the patient to sympathetic nervous system stimulation, such as cold temperature, for BAT to be activated and detectable. The problem with the background FDG uptake in tissues other than BAT that also have high metabolic activity would also be overcome. Besides, the alternative methodology could be cheaper and more generally available, as PET scanners are not routinely accessible to clinical practitioners. Finally, by comparing BAT mass with functional BAT activity (assessed by PET) new physiological mechanisms of BAT activation could be discovered. Previous research had laid out the foundation for non-invasive methods to image BAT with fluorescent dyes;Citation16 however, the lack of BAT-specific probes has limited their usefulness.
BAT Imaging through Peptide-Guided Probes
Because current methods for BAT detection rely on the metabolic activity of this tissue, analyzing the abundance and biodistribution of dormant BAT in the body has remained challenging. Over the past decade, phage-display libraries have been screened for identification of peptides targeting organ-selective cell surface receptors.Citation17-Citation20 Based on the notion that angiogenic tumor vasculature features differential expression of endothelial markers, our group has performed screens in live animals and end-of-life patients to isolate peptides that selectively home to blood vessels of individual organs.Citation17,Citation21,Citation22 In a recent study, we used this approach to screen a combinatorial library for peptides localizing to BAT upon systemic administration.Citation23 With the goal of establishing new approaches for BAT detection in the body, we analyzed BAT-homing capacity of a panel of isolated peptides. We validated a phage-displayed peptide (sequence CPATAERPC), termed PEP3, that selectively binds to the vascular endothelium of BAT, but not of visceral WAT (). Through tissue section analysis, we demonstrated that PEP3, when coupled with a with the Cy3 fluorophore, can be used as a probe identifying BAT depots. We showed that, in addition to BAT, this peptide probe recognizes the vasculature of BAT-like depots of subcutaneous (sc) WAT.
Figure 1. Fluorescence on sectioned mouse tissue showing blood vessels (green) labeled with peptide PEP3 (red) targeting brown adipose tissue. Nuclei (blue) reveal brown adipocytes.
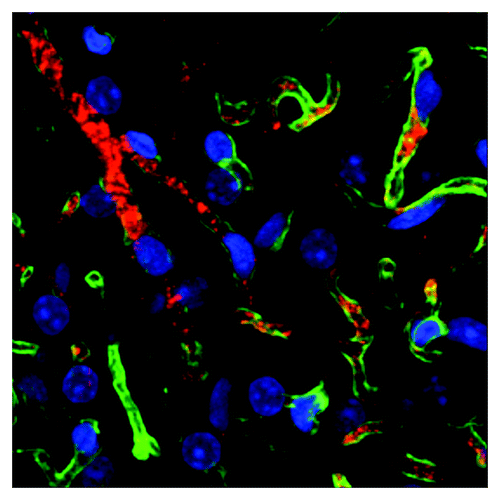
We also tested the peptide in near-infrared (NIR) fluorescence imaging, which gives the advantage of comparatively high tissue penetration and low background.Citation24 IRDye800, a fluorophore that emits in the NIR range, was conjugated with PEP3 via amide linkage to a lysine residue inserted at the N-terminus.Citation25 By analyzing resected tissue upon peptide systemic administration, we demonstrated that PEP3-IRDye800 selectively accumulated in BAT, while a previously reported WAT-homing peptideCitation18 had a preference for WAT. We showed that PEP3 localizes to BAT endothelium without cold acclimation and UCP1 upregulation, and even upon treatment with a β blocker propranolol. These results indicate that PEP3 can localize to BAT irrespective of tissue metabolic activity, although increased PEP3 accumulation in BAT of cold-stimulated animals suggests that metabolic activation does increase PEP3 receptor bioavailability. We also confirmed PEP3-IRDye800 preferentially accumulates in BAT through non-invasive whole body imaging. This report is a proof of concept that BAT can be detected via ligand-directed fluorescent probe delivery. It should be noted that the peptide design in our study included the chelation moiety 1,4,7-triazacyclononane-N,N',N''-triacetic acid (NODAGA) which could be used for dual labeling of fluorescent peptides with a radioisotope in future applications.
Shades of Brown: the Diversity of Adipocytes
Over the past few years, it has become clear that, in addition to bona fide BAT present in infancy, both mice and humans have a variable brown-like component in WAT.Citation26 Under certain conditions, white adipocytes can become replaced with brown-like (beige/brite) adipocytes that simulate the adaptive thermogenesis function of BAT adipocytes.Citation27 Lineage tracing studies in mice based on the Myf5 promoter indicated that brown adipocytes associated with the muscle tissue are derived from the same precursors as myocytes.Citation28,Citation29 However, as a part of these fate-mapping studies, it has also been demonstrated that brown adipocytes located within residual WAT are derived from different Myf5-negative progenitors, suggesting precursors of white adipocytes as another origin of BAT.Citation30 Consistent with this possibility, adipose stromal cells (ASC), which we and others have shown to serve as white adipocyte progenitors,Citation4,Citation13,Citation31 can also be differentiated into brown adipocytes.Citation32 According to Cinti and colleagues, differentiated cells of WAT can be directly converted to a brown adipocyte-like phenotype through hyperleptinemia.Citation33 Analyses of mouse BAT patches within WAT based on electron microscopy and BrdU incorporation have suggested that white adipocytes can undergo reversible transdifferentiation into brown adipocytes.Citation34 This conversion is driven by sympathetic nervous system stimuli, such as cold temperature, and signal transduction cascades triggered by activation of β-3 adrenergic receptors in WAT.Citation35 The resulting areas of beige adipocytes with the characteristic BAT mitochondrial and lipid droplet architecture express UCP1 and cluster around bundles of blood vessels and nerves.Citation34 Prolonged exposure of mice to cold temperature results in progressing expansion of these patches leading to virtually all adipose depots becoming BAT-like at the expense of WAT. Adipose tissue interconversion phenomenon has been confirmed by lineage tracing studies based on UCP1 promoter.Citation36
A recent lineage tracing study demonstrates that a large proportion of brown-like adipocytes within WAT depots arises from progenitors de novo upon β-3 adrenergic stimulation.Citation37 The contribution of progenitors to adipocytes during development and WAT remodeling in obesity or adrenergic stimulation is depot-specific.Citation37-Citation39 While re-differentiation of adipocytes may explain WAT browning in some cases, a growing body of evidence indicates that white and brown-like adipocytes in WAT can arise from distinct progenitor populations. White adipocyte progenitors have been shown to express platelet-derived growth factor receptor β (PDGFRβ) and delta-decorin (ΔDCN), a proteolytic cleavage fragment of decorin.Citation40 Recently, PDGFRα was revealed as a marker of progenitors capable of differentiating not only into white adipocytes,Citation41 but also into beige adipocytes in WAT.Citation41,Citation42
In light of the apparent brown adipocyte diversity, the question concerning any imaging study is whether “constitutive” or “recruited” BAT is being imaged. In fact, recent reports suggest that tissue identified as BAT by PET in humans may contain both truly brown and beige adipocytes.Citation43 Although human BAT appears to be more similar to mouse beige fat than mouse BAT,Citation27 “beiging” of white fat in humans remains to be demonstrated.Citation44 While PEP3, the BAT-homing probe described in our study,Citation23 does not home to intraperitoneal WAT, it does home to metabolically active beige adipose tissue found within sc WAT. The conservation of endothelial markers in the vasculature of BAT and sc WAT revealed via PEP3 is not surprising in light of the recent studies indicating the common origin of BAT and of certain sc WAT depots.Citation38 Because PEP3 also localizes to vasculature in browning areas of sc WAT, and its accumulation in both BAT and sc WAT increases upon sympathetic nervous system stimulation, it is currently not entirely clear to what extent metabolic activity is a requisite for PEP3 homing. In any event, peptide-based imaging techniques may provide a means of identifying whether there are pockets of human WAT beiging. Although determining the adipocyte identity in various depots imaged with targeted probes will have academic value, it may matter little clinically if it turns out that all types of brown adipocytes engage in electron chain uncoupling and thermogenesis.
Prospects in Adipose Tissue Imaging
Currently practiced PET imaging technology can detect only metabolically active BAT and is prone to false positive signals from other metabolically active tissues.Citation45,Citation46 There are a number of challenges to be considered for evolving imaging applications to be successful. An intrinsic problem with imaging adipose tissue is that a layer intradermal adipocytes coats most of the body resulting in high peripheral signal. This matter is further complicated in certain locations by localized subcutaneous fat depots, which makes it difficult to image internal tissues by many optical imaging approaches. Because beige adipocytes are mostly found within the subcutaneous adipose depots, their analysis is plagued with the same problems. For whole body BAT detection to be successful, imaging platforms not amenable to this limitation need to be used, and there is progress being made.Citation47 However, for every new imaging approach important questions remain to be addressed. How much BAT (in terms of brown adipocyte number in a cluster) within the surrounding tissue is there enough for detection? How close to the body surface do they need to be in order to be detected? What are the depot-specific background signals or interferences from neighboring organs to be taken into consideration? It will be important to overcome false negative and false positive signal limitations, which will help to validate the emerging techniques in clinical trials.
The discovery and validation of PEP3Citation23 illustrates the potential of the screens for agents useful as imaging probes homing to BAT regardless of tissue activity. It also sets the foundation for future screens that may identify new probes useful for specific detection of individual BAT depots. Targeted peptide probes like PEP3 might enable a way to monitor changes in highly dynamic body BAT/WAT distribution. Eventually, BAT-homing agents could be used for the development of vectors aimed at stimulating residual BAT. This potentially could evolve into new obesity treatments based on shifting the balance in adipose tissue toward its conversion to metabolically active state. New generation probes enabling a more specific targeting of each individual type of adipose tissue found in the body will also be anticipated. While screens of peptide libraries for molecular “zip codes” typically identify selectively expressed endothelial receptors,Citation18 perivascular cells are also accessible to circulation in adipose tissue and peptides targeting ASC have been reported.Citation20,Citation40 Therefore, future efforts could identify probes for BAT stromal cells, or possibly even adipocytes, that could be administered systemically. Possibly overcoming problems associated with the diversity of stromal progenitors and adipose tissue depots remodeled through their recruitment, combinations of probes for distinct subpopulations of adipose cells could enable mapping of tissue “browning” in development or disease.
The translational objective of the current efforts is the eventual development of probes for imaging BAT in humans. Potential applications are numerous. For example, imaging technologies of the future could become useful for monitoring BAT stimulation as a read-out of the patient’s progress upon obesity treatment. It remains to be tested whether PEP3 binds to a receptor expressed in human BAT vasculature and could be used for clinical ligand-guided imaging. Organ-homing peptides can be used as “baits” for isolation of the corresponding biological receptors.Citation21,Citation40 Identification of the cell surface receptor for PEP3 might lead to the discovery of new molecular networks operating in the context of BAT vasculature, with implications for our understanding of adipose biology and for drug discovery. The established approaches to perform peptide screens directly in humansCitation17,Citation48 may expedite the progress in the field.
As new BAT detection strategies are improved, their future applications might enable personalize treatments based on brown adipocyte quantities and distribution in patients. Because aging and obesity, resulting from excess accumulation of WAT, are associated with reduction in BAT, it has been proposed that obesity development may be suppressible by the amount and/or activity of BAT.Citation10,Citation33,Citation34 Recent studies in mouse models support this concept, which opens new perspectives for obesity management.Citation49 It must be noted, however, that inducing BAT activity in adult humans results in a relatively minor increase in energy expenditure according to a recent study.Citation44 This indicates that BAT activation may be insufficient for significant effects on weight, pointing to potential therapeutic limitations. Moreover, thermogenic lypolysis can also be implicated in pathogenesis of life-threatening conditions.Citation50 In conclusion, enabling of BAT imaging in disease is of high importance.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by NIH NIDDK grant 1R21DK090752 to M.G.K. I thank Alexes Daquinag for providing the image.
References
- Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 2006; 444:847 - 53; http://dx.doi.org/10.1038/nature05483; PMID: 17167472
- Flier JS. Obesity wars: molecular progress confronts an expanding epidemic. Cell 2004; 116:337 - 50; http://dx.doi.org/10.1016/S0092-8674(03)01081-X; PMID: 14744442
- Sirin O, Kolonin MG. Treatment of obesity as a potential complementary approach to cancer therapy. Drug Discov Today 2013; 18:567 - 73; http://dx.doi.org/10.1016/j.drudis.2012.05.008; PMID: 22627005
- Daquinag AC, Zhang Y, Kolonin MG. Vascular targeting of adipose tissue as an anti-obesity approach. Trends Pharmacol Sci 2011; 32:300 - 7; http://dx.doi.org/10.1016/j.tips.2011.01.004; PMID: 21349592
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 2004; 84:277 - 359; http://dx.doi.org/10.1152/physrev.00015.2003; PMID: 14715917
- Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell 2007; 131:242 - 56; http://dx.doi.org/10.1016/j.cell.2007.10.004; PMID: 17956727
- Paidisetty S, Blodgett TM. Brown fat: atypical locations and appearances encountered in PET/CT. AJR Am J Roentgenol 2009; 193:359 - 66; http://dx.doi.org/10.2214/AJR.08.2081; PMID: 19620432
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerbäck S, et al. Functional brown adipose tissue in healthy adults. N Engl J Med 2009; 360:1518 - 25; http://dx.doi.org/10.1056/NEJMoa0808949; PMID: 19357407
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009; 360:1509 - 17; http://dx.doi.org/10.1056/NEJMoa0810780; PMID: 19357406
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med 2009; 360:1500 - 8; http://dx.doi.org/10.1056/NEJMoa0808718; PMID: 19357405
- Almind K, Manieri M, Sivitz WI, Cinti S, Kahn CR. Ectopic brown adipose tissue in muscle provides a mechanism for differences in risk of metabolic syndrome in mice. Proc Natl Acad Sci U S A 2007; 104:2366 - 71; http://dx.doi.org/10.1073/pnas.0610416104; PMID: 17283342
- Lowell BB, S-Susulic V, Hamann A, Lawitts JA, Himms-Hagen J, Boyer BB, Kozak LP, Flier JS. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature 1993; 366:740 - 2; http://dx.doi.org/10.1038/366740a0; PMID: 8264795
- Zeve D, Tang W, Graff J. Fighting fat with fat: the expanding field of adipose stem cells. Cell Stem Cell 2009; 5:472 - 81; http://dx.doi.org/10.1016/j.stem.2009.10.014; PMID: 19896439
- Hany TF, Gharehpapagh E, Kamel EM, Buck A, Himms-Hagen J, von Schulthess GK. Brown adipose tissue: a factor to consider in symmetrical tracer uptake in the neck and upper chest region. Eur J Nucl Med Mol Imaging 2002; 29:1393 - 8; http://dx.doi.org/10.1007/s00259-002-0902-6; PMID: 12271425
- Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 2007; 293:E444 - 52; http://dx.doi.org/10.1152/ajpendo.00691.2006; PMID: 17473055
- Nakayama A, Bianco AC, Zhang CY, Lowell BB, Frangioni JV. Quantitation of brown adipose tissue perfusion in transgenic mice using near-infrared fluorescence imaging. Mol Imaging 2003; 2:37 - 49; http://dx.doi.org/10.1162/153535003765276273; PMID: 12926236
- Arap W, Kolonin MG, Trepel M, Lahdenranta J, Cardó-Vila M, Giordano RJ, Mintz PJ, Ardelt PU, Yao VJ, Vidal CI, et al. Steps toward mapping the human vasculature by phage display. Nat Med 2002; 8:121 - 7; http://dx.doi.org/10.1038/nm0202-121; PMID: 11821895
- Kolonin M, Pasqualini R, Arap W. Molecular addresses in blood vessels as targets for therapy. Curr Opin Chem Biol 2001; 5:308 - 13; http://dx.doi.org/10.1016/S1367-5931(00)00207-6; PMID: 11479123
- Sergeeva A, Kolonin MG, Molldrem JJ, Pasqualini R, Arap W. Display technologies: application for the discovery of drug and gene delivery agents. Adv Drug Deliv Rev 2006; 58:1622 - 54; http://dx.doi.org/10.1016/j.addr.2006.09.018; PMID: 17123658
- Kolonin MG. Tissue-specific targeting based on markers expressed outside endothelial cells. Adv Genet 2009; 67:61 - 102; http://dx.doi.org/10.1016/S0065-2660(09)67003-6; PMID: 19914450
- Kolonin MG, Saha PK, Chan L, Pasqualini R, Arap W. Reversal of obesity by targeted ablation of adipose tissue. Nat Med 2004; 10:625 - 32; http://dx.doi.org/10.1038/nm1048; PMID: 15133506
- Kolonin MG, Sun J, Do KA, Vidal CI, Ji Y, Baggerly KA, Pasqualini R, Arap W. Synchronous selection of homing peptides for multiple tissues by in vivo phage display. FASEB J 2006; 20:979 - 81; http://dx.doi.org/10.1096/fj.05-5186fje; PMID: 16581960
- Azhdarinia A, Daquinag AC, Tseng C, Ghosh S, Amaya-Manzanares F, Sevick-Muraca E, Kolonin MG. A peptide probe for targeted brown adipose tissue imaging. Nature Comm 2013.
- Sevick-Muraca EM. Translation of near-infrared fluorescence imaging technologies: emerging clinical applications. Annu Rev Med 2012; 63:217 - 31; http://dx.doi.org/10.1146/annurev-med-070910-083323; PMID: 22034868
- Ghosh SC, Ghosh P, Wilganowski N, Robinson H, Hall MA, Dickinson G, Pinkston KL, Harvey BR, Sevick-Muraca EM, Azhdarinia A. Multimodal chelation platform for near-infrared fluorescence/nuclear imaging. J Med Chem 2013; 56:406 - 16; http://dx.doi.org/10.1021/jm300906g; PMID: 23214723
- Cinti S. Between brown and white: novel aspects of adipocyte differentiation. Ann Med 2011; 43:104 - 15; http://dx.doi.org/10.3109/07853890.2010.535557; PMID: 21254898
- Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012; 150:366 - 76; http://dx.doi.org/10.1016/j.cell.2012.05.016; PMID: 22796012
- Atit R, Sgaier SK, Mohamed OA, Taketo MM, Dufort D, Joyner AL, Niswander L, Conlon RA. Beta-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev Biol 2006; 296:164 - 76; http://dx.doi.org/10.1016/j.ydbio.2006.04.449; PMID: 16730693
- Kajimura S, Seale P, Kubota K, Lunsford E, Frangioni JV, Gygi SP, Spiegelman BM. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature 2009; 460:1154 - 8; http://dx.doi.org/10.1038/nature08262; PMID: 19641492
- Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scimè A, Devarakonda S, Conroe HM, Erdjument-Bromage H, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008; 454:961 - 7; http://dx.doi.org/10.1038/nature07182; PMID: 18719582
- Traktuev DO, Merfeld-Clauss S, Li J, Kolonin M, Arap W, Pasqualini R, Johnstone BH, March KL. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res 2008; 102:77 - 85; http://dx.doi.org/10.1161/CIRCRESAHA.107.159475; PMID: 17967785
- Elabd C, Chiellini C, Carmona M, Galitzky J, Cochet O, Petersen R, Pénicaud L, Kristiansen K, Bouloumié A, Casteilla L, et al. Human multipotent adipose-derived stem cells differentiate into functional brown adipocytes. Stem Cells 2009; 27:2753 - 60; http://dx.doi.org/10.1002/stem.200; PMID: 19697348
- Orci L, Cook WS, Ravazzola M, Wang MY, Park BH, Montesano R, Unger RH. Rapid transformation of white adipocytes into fat-oxidizing machines. Proc Natl Acad Sci U S A 2004; 101:2058 - 63; http://dx.doi.org/10.1073/pnas.0308258100; PMID: 14769942
- Cinti S. Transdifferentiation properties of adipocytes in the adipose organ. Am J Physiol Endocrinol Metab 2009; 297:E977 - 86; http://dx.doi.org/10.1152/ajpendo.00183.2009; PMID: 19458063
- Enerbäck S. The origins of brown adipose tissue. N Engl J Med 2009; 360:2021 - 3; http://dx.doi.org/10.1056/NEJMcibr0809610; PMID: 19420373
- Rosenwald M, Perdikari A, Rülicke T, Wolfrum C. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol 2013; 15:659 - 67; http://dx.doi.org/10.1038/ncb2740; PMID: 23624403
- Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med 2013; 19:1338 - 44; http://dx.doi.org/10.1038/nm.3324; PMID: 23995282
- Sanchez-Gurmaches J, Hung CM, Sparks CA, Tang Y, Li H, Guertin DA. PTEN loss in the Myf5 lineage redistributes body fat and reveals subsets of white adipocytes that arise from Myf5 precursors. Cell Metab 2012; 16:348 - 62; http://dx.doi.org/10.1016/j.cmet.2012.08.003; PMID: 22940198
- Lee YH, Petkova AP, Mottillo EP, Granneman JG. In vivo identification of bipotential adipocyte progenitors recruited by β3-adrenoceptor activation and high-fat feeding. Cell Metab 2012; 15:480 - 91; http://dx.doi.org/10.1016/j.cmet.2012.03.009; PMID: 22482730
- Daquinag AC, Zhang Y, Amaya-Manzanares F, Simmons PJ, Kolonin MG. An isoform of decorin is a resistin receptor on the surface of adipose progenitor cells. Cell Stem Cell 2011; 9:74 - 86; http://dx.doi.org/10.1016/j.stem.2011.05.017; PMID: 21683670
- Berry R, Rodeheffer MS. Characterization of the adipocyte cellular lineage in vivo. Nat Cell Biol 2013; 15:302 - 8; http://dx.doi.org/10.1038/ncb2696; PMID: 23434825
- Lee YH, Petkova AP, Granneman JG. Identification of an adipogenic niche for adipose tissue remodeling and restoration. Cell Metab 2013; 18:355 - 67; http://dx.doi.org/10.1016/j.cmet.2013.08.003; PMID: 24011071
- Lidell ME, Betz MJ, Dahlqvist Leinhard O, Heglind M, Elander L, Slawik M, Mussack T, Nilsson D, Romu T, Nuutila P, et al. Evidence for two types of brown adipose tissue in humans. Nat Med 2013; 19:631 - 4; http://dx.doi.org/10.1038/nm.3017; PMID: 23603813
- van der Lans AA, Hoeks J, Brans B, Vijgen GH, Visser MG, Vosselman MJ, Hansen J, Jörgensen JA, Wu J, Mottaghy FM, et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest 2013; 123:3395 - 403; http://dx.doi.org/10.1172/JCI68993; PMID: 23867626
- Tatsumi M, Engles JM, Ishimori T, Nicely O, Cohade C, Wahl RL. Intense (18)F-FDG uptake in brown fat can be reduced pharmacologically. J Nucl Med 2004; 45:1189 - 93; PMID: 15235065
- Fueger BJ, Czernin J, Hildebrandt I, Tran C, Halpern BS, Stout D, Phelps ME, Weber WA. Impact of animal handling on the results of 18F-FDG PET studies in mice. J Nucl Med 2006; 47:999 - 1006; PMID: 16741310
- Gilsanz V, Hu HH, Kajimura S. Relevance of brown adipose tissue in infancy and adolescence. Pediatr Res 2013; 73:3 - 9; http://dx.doi.org/10.1038/pr.2012.141; PMID: 23090604
- Staquicini FI, Cardó-Vila M, Kolonin MG, Trepel M, Edwards JK, Nunes DN, Sergeeva A, Efstathiou E, Sun J, Almeida NF, et al. Vascular ligand-receptor mapping by direct combinatorial selection in cancer patients. Proc Natl Acad Sci U S A 2011; 108:18637 - 42; http://dx.doi.org/10.1073/pnas.1114503108; PMID: 22049339
- Tam CS, Lecoultre V, Ravussin E. Brown adipose tissue: mechanisms and potential therapeutic targets. Circulation 2012; 125:2782 - 91; http://dx.doi.org/10.1161/CIRCULATIONAHA.111.042929; PMID: 22665886
- Dong M, Yang X, Lim S, Cao Z, Honek J, Lu H, Zhang C, Seki T, Hosaka K, Wahlberg E, et al. Cold exposure promotes atherosclerotic plaque growth and instability via UCP1-dependent lipolysis. Cell Metab 2013; 18:118 - 29; http://dx.doi.org/10.1016/j.cmet.2013.06.003; PMID: 23823482
