Abstract
Environmental pollutants, such as bisphenol A (BPA), have the potential to affect the differentiation processes and the biology of the adipose tissue. The 3T3-L1 model is one of the murine cell models used extensively for the investigation of the molecular events that govern the differentiation of adipocytes from a committed preadipocyte to a mature, lipid laden adipocyte. Most of the studies investigating the effects of BPA on preadipocyte differentiation have investigated the effects of this chemical in the presence of an optimal differentiation cocktail containing high concentrations of the synthetic glucocorticoid dexamethasone, conditions that result in 90% to 100% of differentiated adipocytes. Our studies employed the 3T3-L1 cell model in the absence of exogenous glucocorticoids. We show that BPA is able to increase the differentiation of the 3T3-L1 cells under these conditions. Furthermore, the effect of BPA was observed in the absence of the synthetic glucocorticoid (dexamethasone), a hormone known to be required for the differentiation of the 3T3-L1 cells. In addition, BPA upregulated the mRNA expression and protein levels of the terminal marker of adipogenesis the fatty acid binding protein (aP2) in these cells. Interestingly, the known modulators of adipogenesis such as the peroxisome proliferator-activated receptor (PPAR) γ or CCAAT enhancer binding protein (C/EBP) α were not elevated at the mRNA or protein level in response to BPA. Furthermore, BPA upregulated the expression levels of the marker of adipogenesis aP2, through an effect on the transcriptional activity of C/EBPδ and the glucocorticoid receptor (GR) at its promoter.
Introduction
The dramatic increase in the rate of obesity in the Western world, particularly among young children, is a significant public health problem which threatens to reverse the gains in health and longevity achieved in the previous century.Citation1 While diet and a sedentary life-style have clearly contributed to the increasing rate of obesity, there is growing evidence to suggest that exposure to environmental chemicals, known now as “obesogens” may be contributing to this epidemic.Citation2,Citation3 Bisphenol A (BPA) is a chemical widely used as a monomer in polycarbonate plastics, in the epoxy lining of cans and in various epoxy sealants including some used in dentistry.Citation4 Due to BPA’s wide usage in consumer products human exposure to the chemical is ubiquitous. Epidemiological studies have shown detectable levels of BPA in urine and serum of individuals with concentrations reaching as high as 60 ng/mL and disturbingly, even higher in newborns and small children.Citation5 BPA is a suspected obesogen based on a number of studies both in vitro and in vivo.Citation6,Citation7 Furthermore, epidemiological studies have correlated BPA concentrations in urine with cardiovascular disease and type 2 diabetes, and in animal models, pregnant mice exposed to BPA exhibited glucose intolerance, insulin resistance, and the offspring mice having increased weight and insulin resistance later in life.Citation8,Citation9 BPA is unlikely to act as a classical estrogen due to the high concentrations required for the transactivation of the ER by this chemical.Citation10 Nevertheless, several studies have shown that BPA has effects at concentrations within the human exposure range.Citation11,Citation12
The formation of the adipose tissue is dependent upon two processes, one is the determination of the stem cells to the adipose lineage to become preadipocytes and the other is the maturation of the preadipocytes into cells that store lipids, secrete adipokines, and are insulin sensitive.Citation13 The molecular mechanisms that control adipogenesis have been extensively studied in cell models such as the committed preadipocyte murine cell line 3T3-L1. In this cell line the differentiation process is tightly regulated by a number of transcription factors such as the CCAAT-enhancer-binding protein (C/EBP) family of transcription factors C/EBPα, β, and δ, and the peroxisome–proliferator-activated receptor (PPAR) γ. Environmental pollutants, such as BPA, have the potential to affect the adipose differentiation processes at several key points.Citation14-Citation16 Indeed, several studies in the literature have provided evidence that BPA can affect the differentiation process of preadipocytes.Citation6,Citation17,Citation18 Recent studies also proposed mechanisms of action of BPA such as GR activation and upregulation of the 11β-hydroxysteroid dehydrogenase (HSD) 1 enzyme.Citation17-Citation19
This study was initiated in order to evaluate the effect BPA on the differentiation of the 3T3-L1 cells and to determine the mechanism of action of this chemical. We found that BPA was able to induce the mRNA and protein levels of the adipogenic marker aP2 in the absence of dexamethasone. Moreover, this is one of the first studies showing that the upregulation of aP2 by BPA is mediated at least partially through a direct effect on the aP2 promoter via the enhancement of the transcriptional activity of C/EBPδ in the presence of GR, a new mechanism of action for BPA.
Results
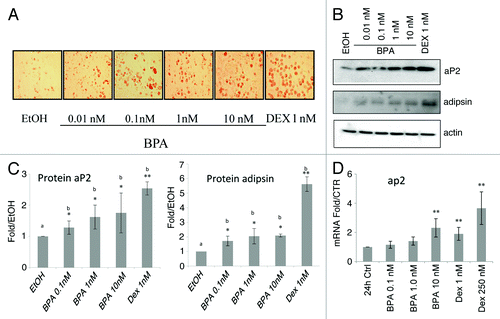
Bisphenol A induces aP2 and adispin expression, and lipid accumulation in 3T3-L1 preadipocytes
We set out to investigate whether BPA can affect the differentiation of the 3T3-L1 preadipocytes in the presence or absence of glucocorticoids and to elucidate the mechanism of BPA action. In order to assess the ability of BPA to affect the differentiation of the 3T3-L1 preadipocytes we visualized lipid accumulation on day 8 at the end of the differentiation process by staining with oil Red O. The data show that treatment of 3T3-L1 cells with BPA induces lipid accumulation at all concentrations used compared with control (). Next, we examined the ability of BPA to induce the expression of aP2, and adipsin which are established markers of mature adipocytes in 3T3-L1 cells. Our results show a dose response after BPA treatment in aP2 protein levels reaching a 2.5-fold increase at 10 nM BPA. Adipsin protein levels were also upregulated reaching a 2-fold upregulation at 1 and 10 nM BPA (, upper panel and lower panel respectively). Using real-time PCR analysis a 2.3-fold upregulation in mRNA levels of aP2 were observed after treatment of the cells with 10 nM BPA. As a positive control the cells were treated with DEX at low (1 nM) and high (250 nM) concentrations which resulted in an increase of 1.9 and 3.6-fold respectively in aP2 mRNA (). These results were obtained from five independent experiments performed in triplicates.
Figure 1. Bisphenol A (BPA) treatment of 3T3-L1 cells induces adipocyte differentiation markers and lipid accumulation. (A) Oil red O lipid staining of 3T3-L1 cells at day 8 treated with the indicated concentrations of BPA. (B) Western blot analyses of aP2 and adipsin at day 8. (C) Fold over MI ethanol control of the western blots normalized to β-actin levels and quantified with BioRad ImageLab software (n = 3). (D) aP2 mRNA levels over control normalized to β-actin mRNA after 24 h treatment (n = 5). Error bars indicate SD (*P < 0.05, **P < 0.01).
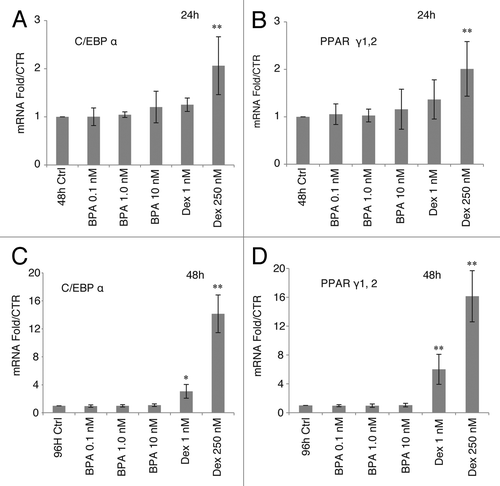
BPA upregulation of aP2 occurs in the absence of upregulation of C/EBPα or PPARγ mRNA
The mRNA level of aP2 in the 3T3-L1 cells is regulated by the transcription factors of the C/EBP family members and PPARγ.Citation20 The levels of mRNA for C/EBPα and PPARγ were unchanged in response to BPA treatments compared with ethanol controls at 24 h () or 48 h (). Treatment with low DEX (1 nM) or high DEX (250 nM) resulted in a robust upregulation in the mRNA levels of C/EBPα at 48 h by 3-fold and 14-fold respectively, and PPARγ mRNA by 6- and 16-fold respectively ().
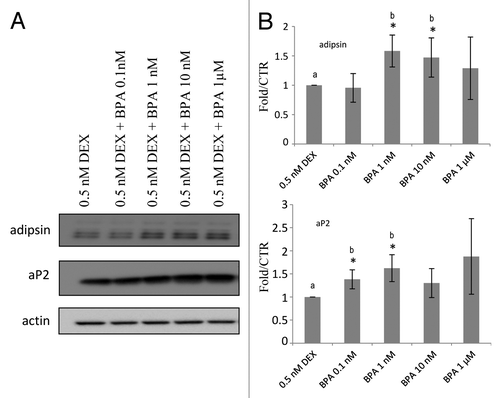
BPA enhances low DEX upregulation of aP2 and adipsin in 3T3L1 cells
Next we investigated whether BPA-induced differentiation is potentiated by low concentrations of glucocorticoids. The cells were co-treated with BPA and low concentrations of DEX as indicated in and aP2 protein levels were determined at day 8 by western blot analysis. Concentrations of 1, 10, and 1000 nM BPA were able to increase the protein levels of aP2 by 50% as compared with the cells treated with DEX alone (). These results suggest that BPA potentiates the ability of DEX to enhance the differentiation of the cells.
Figure 3. BPA upregulation of aP2 expression is potentiated by low dose DEX treatment.(A) Western blot analyses of aP2 in the presence of low DEX. (B) Fold over MI ethanol control of the western blots normalized to β-actin levels and quantified with BioRad ImageLab software (n = 3). Error bars indicate SD *P < 0.05 (a vs. b).
BPA does not act as a transcriptional activator of the glucocorticoid receptor on the MMTV or C/EBPα promoters
Our results thus far indicated that BPA can induce differentiation of 3T3-L1 cells in the absence of DEX (). In an effort to understand the molecular mechanism by which BPA potentiates the differentiation of the 3T3-L1 cells we investigated whether it can act as a glucocorticoid and activate the glucocorticoid receptor. To address this hypothesis we performed transcription assays using three different glucocorticoid responsive promoters driving the luciferase gene expression when transiently transfected into NIH 3T3 cells. The three promoters tested were: a synthetic glucocorticoid response element (3X-GRE), the mouse mammary tumor virus (MMTV) promoter (highly responsive to glucocorticoid treatment), and the C/EBPα promoter (a non-GRE containing glucocorticoid responsive promoter). C/EBPα is a DEX target and one of the transcription factors upregulated after the initiation of differentiation in the 3T3-L1 cells and has been shown to upregulate aP2 expression.Citation21,Citation22 As expected, both the synthetic and the MMTV containing GRE promoters were responsive to DEX treatment resulting in an increase in luciferase activity of more than 1000-fold for the MMTV promoter () and 2- to 3-fold when the target promoter was the 3X-GRE-Luc (). However, treatment of the cells with BPA did not result in an increase in the luciferase activity suggesting no direct effect on the transactivation of the glucocorticoid receptor on these promoters. We have previously shown that GR potentiates the transcriptional activity of the C/EBPβ transcription factor at the C/EBPα promoter through the exchange of transcriptional co-repressors for transcriptional activators at the promoter, in the absence of direct binding of GR to the DNA.Citation23,Citation24 Next, we wanted to determine whether BPA affects the transcriptional activity of GR through effects on transcriptional complexes that are present at the C/EBPα promoter. NIH 3T3 cells transiently transfected with the C/EBPα-Luc construct in conjunction with C/EBPβ and GR expression vectors have been shown to upregulate the C/EBPα promoter.Citation23 Although a clear effect of DEX treatment was observed (4-fold increase over control), no effect of BPA could be detected at this promoter even at concentrations as high as 50 μM (, and data not shown).
Figure 4. BPA treatment does not result in transcriptional transactivation of the glucocorticoid receptor on the MMTV or the C/EBPα promoters.(A) Relative luciferase activity (RLU) normalized to pRL-CMV of pTL2-GR on the MMTV promoter, 16 h after treatment with the indicated concentrations of BPA or 250 nM dexamethasone (DEX). Graphs show average relative luciferase units (RLU) (n = 3). (B) As in (A) with the reporter plasmid being pGRE-Luc (n = 3). (C) Relative luciferase activity (RLU) normalized to pRL-CMV activity of C/EBPβ and pTL2-GR on the C/EBPα-Luc promoter (n = 3). (D) As in (C) after co-treatment with the indicated concentrations of BPA and 0.5 nM DEX (n = 3). Error bars indicate SD (*P < 0.05, **P < 0.01).
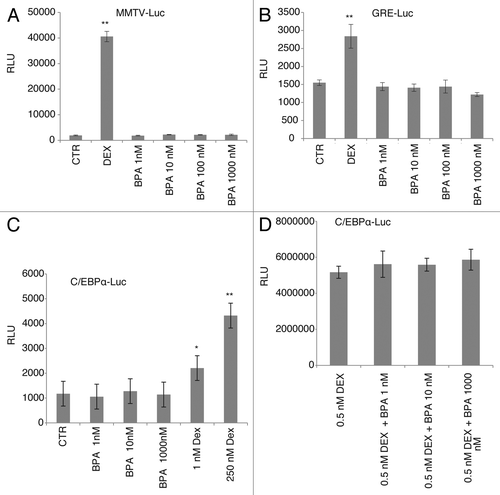
Based on the results that BPA has an additive effect on aP2 expression in the presence of low concentrations of glucocorticoids () we further investigated whether BPA requires the presence of glucocorticoids for its action. The luciferase expression induced by a sub-maximally-active concentration of DEX (0.5 nM), which on its own resulted in a 2-fold increase in luciferase activity (data not shown), was not further increased by co-treatment with any dose of BPA ().
Together these results indicate that although BPA has a clear effect on the expression of terminal differentiation markers of adipogenesis such as aP2 in the 3T3-L1 cells it does not transactivate GR on the MMTV, GRE-Luc, or the C/EBPα promoter.
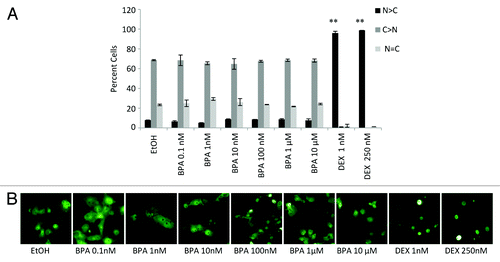
An additional level of GR regulation is achieved via the regulation of the cellular distribution of the receptor. Where GR is localized in the cytoplasm in a transcriptionally inactive complex it can be transferred to the nucleus in response to extracellular stimuli other than ligand binding such as shear stress.Citation25-Citation27 Therefore, one can hypothesize that BPA may act on a signaling pathway that can in turn promote the nuclear translocation of GR, and perhaps increase its activity in the cell by increasing the GR concentration in the nucleus. Cos-7 cells, which do not express endogenous GR, were transiently transfected with a GFP-GR expression vector and the localization of the receptor was visualized by fluorescence microscopy post treatment with BPA or DEX at the indicated concentrations for 16 h (). A robust response in the nuclear translocation of the receptor was obtained after treatment with DEX at all concentrations tested. In contrast, no difference in the nuclear localization of the receptor was observed after treatment of the cells with various concentrations of BPA reaching 50 μM (, data not shown, and visualized in ). This suggests that BPA is not acting like a classical glucocorticoid in our cell model.
Figure 5. Nuclear localization of GFP-GR after BPA and DEX treatment. (A) Localization GFP-GR expressed in COS-7 cells before and after 6 h treatment with the indicated concentration of BPA or DEX determined by direct fluorescence. (A) Quantifications of at least three independent experiments performed in duplicate where at least 200 cells were counted per slide. Error bars indicate the SD (**P < 0.01). (B) Representative micrographs are shown below each data set.
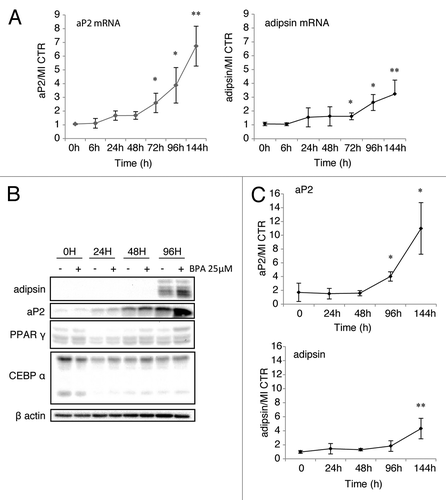
High doses of BPA upregulate the expression of aP2 and adipsin at the protein and mRNA levels in the absence of glucocorticoids and in the presence of insulin
In an effort to identify the mechanism of action of BPA on the adipogenic potential of the 3T3-L1 cells we have used several conditions and have found that at 25 μM BPA had an optimal effect when the cells were treated first with IBMX and insulin for the first 48 h followed by insulin for the remaining differentiation process in the presence of the chemical. The RNA levels were increased after BPA treatment by 8-fold for aP2 mRNA and 3-fold for adipsin (). Similarly, protein levels of aP2 and adipsin were increased by BPA treatment by 10-fold and 4-fold respectively (). These effects were observed in the presence of IBMX and insulin alone, and in the absence of 250 nM dexamethasone, which upregulated the expression levels of aP2 by 30-fold (data not shown). Interestingly, the effect on adipsin expression was much more modest (4-fold) as compared with the MI control. It is worth mentioning that BPA was able to upregulate the expression of aP2 also in the absence of insulin or IBMX, however the overall levels were very low (data not shown). This pronounced effect on the expression levels of aP2 was achieved in the absence of upregulation of PPARγ or C/EBPα as shown in middle panels, factors that are extremely responsive to the glucocorticoid treatment (data not shown and ref. Citation28) aP2 was increased both at the protein and the mRNA levels in what seems to be a direct transcriptional effect on the aP2 gene since it occurred as early as 24 h after BPA treatment (). Adipsin mRNA was also upregulated by BPA treatment however, the effects were much less pronounced reaching only 3-fold after 144 h treatment.
Figure 6. High dose BPA upregulates aP2 and adipsin in the absence of PPARγ or C/EBPα induction.(A) mRNA levels of aP2 (left panel) and adipsin (right panel) standardized against control β-actin mRNA levels and expressed as fold over MI + vehicle control at the indicated time points (n = 5). (B) Protein levels of the indicated proteins by western blot. (C) Analysis of western blots of aP2 and adipsin protein levels quantified using the BioRad ImageLab software represented as fold increase of aP2 or adipsin respectively, over MI + vehicle and normalized to β-actin protein levels (n = 3). Error bars represent SD (*P < 0.05, **P < 0.01).
BPA increases the transcriptional activity of the glucocorticoid receptor and C/EBPδ on the aP2 promoter
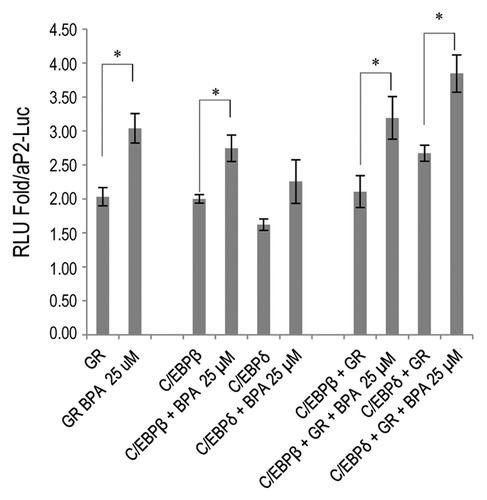
aP2 transcription is known to be mediated by a number of transcription factors such as PPARγ, C/EBPα, and C/EBPδ.Citation29 The expression levels of all of these factors were not changed in response to BPA treatment either at the protein or the mRNA level (, and data not shown). Therefore, we proceeded to investigate whether BPA than upregulates the transcriptional activity of transcription factors known to transactivate the aP2 promoter, without upregulating their expression levels. We therefore cloned the proximal aP2 promoter first described by Wu et al.Citation30 into the PGL4.1-Luc2 vector and used it to delineate the molecular mechanism by which BPA upregulates the expression of aP2. It has been previously shown that BPA is unable to activate PPARγCitation17 and our results using this promoter confirm these results (data not shown). Further analysis of the promoter revealed a number of potential CCAATT enhancer binding protein (C/EBP) sites and a few potential glucocorticoid responsive elements (GREs) in addition to the PPARγ binding sites. We transfected Cos-7 cells with aP2-Luc together with GR alone or in combination with C/EBPβ or C/EBPδ and treated the cells with 25 µM BPA for 24 h. BPA was able to increase aP2-Luc promoter activity by 2-fold. GR transfection also increased the aP2 promoter activity by 2-fold when compared with the luciferase activity of the vector aP2-Luc and BPA treatment further potentiated GR activity to increase luciferase levels to 3-fold over vector control (). Interestingly, BPA was able to slightly increase C/EBP transcriptional activity on this promoter; however, the most pronounced effect of BPA was observed when both GR and C/EBPδ were present which resulted in an increase in luciferase activity to almost 4-fold over vector control (). We did not observe any transactivation of the MMTV or C/EBPα promoters even at 25 µM BPA (data not shown), which indicates that this effect is specific for the aP2 promoter. These results suggest that one of the mechanisms of action of BPA is mediated at through collaboration between GR and members of the C/EBP family members of transcription factors such as C/EBPδ, at specific target promoters.
Figure 7. BPA potentiates the transcriptional activity of GR, C/EBPβ and δ at the aP2 promoter. Cos-7 cells were transfected with aP2-Luc alone, pTL2-GR or pTL2-GR with MSV-C/EBPβ or MSV-C/EBPδ. Graph shows the fold change in luciferase activity over aP2-Luc control. Error bars indicate SD (*P < 0.05).
Discussion
BPA is among the most widely used substance in commerce resulting in near ubiquitous exposure among the human population of the developed world.Citation31 Most absorbed BPA is rapidly conjugated to a glucuronide which is considered to be inactive as an estrogenic compound.Citation32 Although there is no consensus on the exact concentrations of free BPA circulating in the serum of individuals published measures of unconjugated BPA in human fluids ranges from undetectable to 800 nM although there is speculation that the latter measures may be due to post-collection contamination.Citation33,Citation34 The health risks represented by this level of free BPA have been the subject of considerable debate.Citation3 Our studies aimed to investigate the effects of low doses of BPA on adipogenesis, using the murine 3T3-L1 cell line as the model system. Our results show that BPA enhances the adipogenic potential of the 3T3-L1 cells at concentrations as low as 0.1 nM—concentrations within the range reported in humans.Citation3 The effects of BPA at comparably low doses have been reported in adipose tissue where decreased adiponectin production by human explants and adipose tissue were shown after treatment of the explants with picomolar concentrations of BPA,Citation11 and more recently in the 3T3-L1 system.Citation17
Glucocorticoids are known adipogenic factors that enhance adipocyte differentiation both in murine and human model systems.Citation35 Our initial hypothesis was that glucocorticoids may be either required for the action of BPA treatment or that BPA can act in the absence of DEX in the differentiation process. Our data clearly illustrate that BPA does not require the presence of DEX for the potentiation of the differentiation process, as illustrated by the 2-fold increase in the levels of mature adipocyte markers such as aP2 by BPA treatment ( and ). Interestingly, BPA treatment was still able to increase adipogenesis in the presence of low and high concentrations of DEX ( and data not shown) which suggests the activation of a parallel pathway. The transcriptional assays using three different glucocorticoid responsive promoters strongly argue that BPA does not act as a pharmacological glucocorticoid and does not activate the transcriptional activity of the glucocorticoid receptor. In contrast, one report in the literature showed direct activation of the glucocorticoid receptor at the transcriptional level using BPA as the agonist on the highly responsive MMTV promoter.Citation18 We could not show any transcriptional transactivation of the MMTV luciferase reporter plasmid in response to BPA treatment in 3T3-L1 cells, nor in the NIH 3T3 cells either with the transfected receptor or the endogenous receptor. The glucocorticoid receptor is one of the few nuclear receptors that are localized in the cytoplasm in the absence of ligand and upon ligand binding migrates into the nucleus where it activates transcription of target genes.Citation36 Therefore, one of the ways in which BPA could potentiate GR effects would have been through effects on its subcellular localization. Our results could not support such effects as, unlike with treatment with dexamethasone, there was no difference in the subcellular localization of the receptor in response to BPA treatment as evaluated by the subcellular localization of GFP-GR, again reinforcing the notion that BPA is unlikely to act as a classical agonist for the glucocorticoid receptor.
During the adipogenic conversion of the 3T3-L1 cells it is well established that the adipogenesis program is driven by a transcriptional cascade which includes key regulators such as the CCAAT enhancer binding proteins (C/EBP) family of transcription factors and the master of adipogenesis PPARγ.Citation20 In turn, these transcriptional regulators, particularly PPARγ and C/EBPα, are believed to upregulate the expression of proteins that have a role in lipid metabolism such as aP2.Citation37 Our results show that low levels of BPA upregulates aP2 by a magnitude of 2- to 5-fold at the protein level at concentrations as low as 1 nM and as much as 10-fold at the higher concentrations. aP2, also known as fatty acid binding protein 4 (FABP4) is a protein that is expressed in adipocytes and has profound effects on insulin sensitivity and glucose metabolism.Citation29,Citation38 Furthermore, in the aP2−/− apolipoprotein E null mouse model a dramatic reduction in vascular lesions was observed when the mice were exposed to a high fat diet illustrating an important role for this protein in the development of atherosclerosis.Citation39 Therefore, the findings that BPA can upregulate the levels of aP2 in adipocytes are of great importance due to the importance of the levels of this protein in the serum for vascular health. Surprisingly, no increase in the expression levels of the known transcriptional regulators of aP2 expression, such as PPARγ or C/EBPα was observed. Although effects were seen with low BPA concentrations, we felt that in order to investigate the molecular mechanism of action we needed to use concentrations that will give us maximal effects. Such approaches are commonly used for many hormones and other chemicals that are being used at far greater concentrations in in vitro experiments. When BPA was used at high concentrations aP2 expression was increased by at least 10-fold compared with IBMX, insulin, and vehicle treated controls both at the protein and RNA levels. Furthermore, transcription assays using the aP2 promoter showed a 2-fold increase in luciferase activity as compared with the transcriptional activity of GR and C/EBPδ alone, indicating that BPA can potentiate the transcriptional activity of C/EBPδ in the presence of GR. Since C/EBPδ expression is regulated mainly by IBMX and insulin, in the 3T3-L1 model, we expect that this transcription factor will be present in the cells when BPA is added. We have previously shown that GR potentiated the transcriptional activity of C/EBPβ on the C/EBPα promoter and that this was mediated by DEX and interplay between co-activators and co-repressors at the C/EBPα promoter.Citation21,Citation23 Effects on the recruitment of co-activators to promoters in response to treatment with chemicals such as monoethyl-hexyl-phthalate (MEHP) have been previously reported,Citation40 and therefore it is possible that BPA may affect the recruitment of such co-activators to the aP2 promoter in the presence of GR and C/EBPs. We have not seen the same effects on the C/EBPα promoter or the MMTV promoter indicating a level of specificity to the aP2 promoter and not a general glucocorticoid effect. Promoter-specific regulation of transcription factors such as C/EBPβ has been previously reported by us and others, where a specific effect was seen on the C/EBPα promoter but not on the PPARγ promoter or the Mcp1 versus Il6.Citation21,Citation41
In conclusion, we have presented evidence suggesting that concentrations of BPA, similar to the concentrations of free BPA to which humans are exposed, have physiological effects on adipocyte biology. We have shown that BPA can upregulate the expression levels of both aP2 at the mRNA and protein levels in 3T3-L1 preadipocytes. Investigation of the mechanism of action of BPA illustrated that it does not activate GR on the MMTV or the C/EBPα promoters and it is unlikely to act as a PPARγ agonist based on evidence in the literature.Citation17,Citation42 Our data suggests an increase in adipocyte differentiation in response to low concentrations of BPA in the absence of glucocorticoids and the upregulation of the adipogenic marker aP2 through a potentiation of a transcriptional complex containing GR and C/EBPδ specifically on the aP2 promoter. This study is in accordance with numerous other studies that show effects of BPA in both in vivo and in vitro models, including adipose explants and β cellsCitation11,Citation43,Citation44 and presents a novel target of BPA in the adipocyte system.
Materials and Methods
Cell culture and differentiation assays
COS-7 cells (ATCC) were maintained in Dulbecco’s modified Eagle’s medium (Wisent) supplemented with 10% fetal calf serum at 5% CO2. 3T3-L1 and NIH 3T3 cells (ATCC) were maintained in DMEM containing 1.0 g/L glucose supplemented with 10% calf serum (CS) at 10% CO2. For differentiation, 2 d post-confluence, the media were replaced with DMEM with 10% FCS or where indicated with serum-free DMEM media supplemented with 33 µM biotin, 17 µM pantothenate, 10 µg/mL transferrin, 0.2 nM triiodothyronine, and 0.25 mg/mL fetuin (Sigma-Aldrich). Differentiation was induced by treatment with 100 nM insulin (Roche Applied), 3-isobutyl-1-methylxanthine (MIX) (500 µM) (Sigma-Aldrich) and the indicated concentrations of BPA or ethanol as the control. Thereafter, media were replaced every 2 d with serum-free medium containing 100 nM insulin and BPA or ethanol for the remaining 6 d.
Oil red O staining and western blots
Eight days after induction of differentiation the 3T3-L1 cells were fixed with 4% formaldehyde and stained overnight with oil red O (Sigma-Aldrich) as previously described.Citation35,Citation36 For Western analysis, cells were washed in PBS and lysed in modified RIPA buffer, 50 mm Tris (pH 7.4), 150 mM NaCl, 1% IGEPAL, 5 mM EDTA, and protease inhibitor cocktail (Sigma-Aldrich). The lysate was cleared by centrifugation. Equal amounts of protein (10–30 µg) were resolved by SDS-PAGE and transferred to polyvinylidene membrane (Bio-Rad) for western analysis with the primary antibodies for aP2 (adipocyte fatty acid binding protein 4 [FABP4]) (R&D), adipsin (P-16) (Santa Cruz), and β-actin (13E5) (Cell Signaling). Signal intensities of western blots were imaged using the BioRad ChemiDoc Imager and quantified using Bio-Rad Image Lab software. For graphical analysis, data are presented as an average protein induction from at least three independent experiments.
Plasmids
MMTV-Luc, C/EBPα-luciferase reporter gene (−350/+7), pTL2-GR, MSV-C/EBPβ, and MSV-C/EBPδ were previously describedCitation36,Citation45-Citation48 and pRL-CMV was obtained from Promega. The proximal aP2 promoter region was amplified from pBS-aP2 obtained from Addgene (plasmid #11424) described in reference Citation30. The murine aP2 promoter was amplified using the following PCR primers containing KpnI and SacI sites flanking a 1.6 kb region of the proximal aP2 promoter: Forward, GATGGTACCG CACGTCTAAC CCATAG; Reverse, GTGAGCTCTA GTGAGTCGTA TTACGC. The PCR fragment was sub-cloned into the KpnI and SacI sites of the pGL4.10-luc2 vector (Promega) to give rise to aP2-Luc which was verified by DNA sequencing.
Transcription assays
NIH 3T3 or 3T3-L1 cells plated in 12-well cell culture plates in 10% charcoal stripped serum and phenol red free DMEM (Wisent). Twenty-four hours later the cells were transfected with Fugene 6 or Fugene HD (Roche Diagnostics), respectively, according to the manufacturer’s instructions. For GRE-Luc and MMTV-Luc, previously described, the cells were transfected with 125 ng of the reporter plasmid, 25 ng pTL2-GR, and 10 ng pRL-CMV as an internal control. For C/EBPα promoter activity the cells were transfected with 125 ng of the C/EBPα-luciferase reporter gene (−350/+7), 25 ng pTL2-GR, and 25 ng of C/EBPβ cDNA. aP2-Luc activity was assessed using 125 ng of aP2-Luc with 25 ng of pMSV-C/EBPβ cDNA or pMSV-C/EBPδ and 12.5 ng pTL2-GR. In all cases DNA concentrations were brought up to 500 ng/well using sheared salmon sperm DNA (Life Technologies). Following transfection, cells were treated with the indicated concentrations of DEX, BPA or ethanol control for 16–24 h after which the cells were lysed in Passive Lysis Buffer (Promega) and the luciferase activity was assessed using the Dual Luciferase Assay kit (Promega) in a Glomax96 luminometer (Promega).
Real-time PCR
RT PCR Total RNA was extracted from differentiating cells treated as indicated in individual experiments using an RNeasy kit (QIAGEN). RNA (250–500 ng) was reverse transcribed using the QuantiTect® Reverse Transcription kit (QIAGEN) as per the manufacturer’s protocols. For real-time reactions, 5–8 µL of a suitable dilution of cDNA was amplified in a CFX 96 PCR Detection System (BioRad) using the BioRad iQ SYBR Green. The primer pairs for each gene target were: C/EBPα Forward: TGGACAAGAA CAGCAACGAG, Reverse: CCATGGCCTT GACCAAGGAG, aP2 Forward: GGAAGCTTGT CTCCAGTGAA CCATGGCCTT GACCAAGGAG, PPARγ 1/2 Forward: GCCTGCGGAA GCCCTTTGGT Reverse: GCAGTTCCAG GGCCTGCAGC, β-actin: Forward: GACTTCGAGC AAGAGATGGC, Reverse: CCAGACAGCA CTGTGTTGGC. Reactions were standardized against β-actin abundance. All primer efficiencies were higher than 95% and specificity was confirmed by sequence blast and melting curve analysis. Data represent average mRNA abundance from a minimum of three separate experiments.
Subcellular localization
Cos-7 cells were plated on glass coverslips in phenol red free DMEM (Wisent) with 5% charcoal stripped serum (prior to transient transfection with 200 ng pGFP-GRCitation45 using FuGENE 6 [Roche]) as per manufacturer’s instructions. Twenty-four hours post-transfection the cells were treated with the indicated concentrations of DEX and BPA or ethanol control for 6 h. Cells were fixed in 4% paraformaldehyde (Polysciences, Inc.) and mounted on slides with Vectashield 4,6-diamidino-2-phenylindole staining solution (Vector Laboratories). One hundred to two hundred cells were scored per slide in each experiment, by fluorescent microscopy and the data are represented as the average in three independent experiments.
Statistical analyses
Paired Student t tests was performed for single comparisons between two experimental groups and using one-way analysis of variance (ANOVA) followed by the Bonferroni post-test when multiple group comparisons were required. For all statistical analyses, a P value less than 0.05 (P < 0.05) was considered significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Drs SL McKnight (University of Texas Southwestern Medical Center), X-J Yang (McGill University), P Antonson (Karolinska Institutet), and RJG Haché (York University) for plasmids.
Funding
Grant to E.A. from the Chemical Management Plan, Health Canada. J.G.B. is funded through a Natural Sciences and Engineering Research Council of Canada (NSERC) Visiting Scientist Research Fellowship.
References
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA 2006; 295:1549 - 55; http://dx.doi.org/10.1001/jama.295.13.1549; PMID: 16595758
- Heindel JJ, vom Saal FS. Role of nutrition and environmental endocrine disrupting chemicals during the perinatal period on the aetiology of obesity. Mol Cell Endocrinol 2009; 304:90 - 6; http://dx.doi.org/10.1016/j.mce.2009.02.025; PMID: 19433253
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr., Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev 2012; 33:378 - 455; http://dx.doi.org/10.1210/er.2011-1050; PMID: 22419778
- Brotons JA, Olea-Serrano MF, Villalobos M, Pedraza V, Olea N. Xenoestrogens released from lacquer coatings in food cans. Environ Health Perspect 1995; 103:608 - 12; http://dx.doi.org/10.1289/ehp.95103608; PMID: 7556016
- Calafat AM, Weuve J, Ye X, Jia LT, Hu H, Ringer S, Huttner K, Hauser R. Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants. Environ Health Perspect 2009; 117:639 - 44; PMID: 19440505
- Masuno H, Iwanami J, Kidani T, Sakayama K, Honda K. Bisphenol a accelerates terminal differentiation of 3T3-L1 cells into adipocytes through the phosphatidylinositol 3-kinase pathway. Toxicol Sci 2005; 84:319 - 27; http://dx.doi.org/10.1093/toxsci/kfi088; PMID: 15659569
- Somm E, Schwitzgebel VM, Toulotte A, Cederroth CR, Combescure C, Nef S, Aubert ML, Hüppi PS. Perinatal exposure to bisphenol a alters early adipogenesis in the rat. Environ Health Perspect 2009; 117:1549 - 55; http://dx.doi.org/10.1289/ehp.11342; PMID: 20019905
- Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, Melzer D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA 2008; 300:1303 - 10; http://dx.doi.org/10.1001/jama.300.11.1303; PMID: 18799442
- Alonso-Magdalena P, Vieira E, Soriano S, Menes L, Burks D, Quesada I, Nadal A. Bisphenol A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring. Environ Health Perspect 2010; 118:1243 - 50; http://dx.doi.org/10.1289/ehp.1001993; PMID: 20488778
- Gould JC, Leonard LS, Maness SC, Wagner BL, Conner K, Zacharewski T, Safe S, McDonnell DP, Gaido KW. Bisphenol A interacts with the estrogen receptor alpha in a distinct manner from estradiol. Mol Cell Endocrinol 1998; 142:203 - 14; http://dx.doi.org/10.1016/S0303-7207(98)00084-7; PMID: 9783916
- Ben-Jonathan N, Hugo ER, Brandebourg TD. Effects of bisphenol A on adipokine release from human adipose tissue: Implications for the metabolic syndrome. Mol Cell Endocrinol 2009; 304:49 - 54; http://dx.doi.org/10.1016/j.mce.2009.02.022; PMID: 19433247
- Alonso-Magdalena P, Laribi O, Ropero AB, Fuentes E, Ripoll C, Soria B, Nadal A. Low doses of bisphenol A and diethylstilbestrol impair Ca2+ signals in pancreatic alpha-cells through a nonclassical membrane estrogen receptor within intact islets of Langerhans. Environ Health Perspect 2005; 113:969 - 77; http://dx.doi.org/10.1289/ehp.8002; PMID: 16079065
- Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res 2012; 53:227 - 46; http://dx.doi.org/10.1194/jlr.R021089; PMID: 22140268
- Shin BS, Kim CH, Jun YS, Kim DH, Lee BM, Yoon CH, Park EH, Lee KC, Han SY, Park KL, et al. Physiologically based pharmacokinetics of bisphenol A. J Toxicol Environ Health A 2004; 67:1971 - 85; http://dx.doi.org/10.1080/15287390490514615; PMID: 15513896
- Nunez AA, Kannan K, Giesy JP, Fang J, Clemens LG. Effects of bisphenol A on energy balance and accumulation in brown adipose tissue in rats. Chemosphere 2001; 42:917 - 22; http://dx.doi.org/10.1016/S0045-6535(00)00196-X; PMID: 11272914
- Masuno H, Kidani T, Sekiya K, Sakayama K, Shiosaka T, Yamamoto H, Honda K. Bisphenol A in combination with insulin can accelerate the conversion of 3T3-L1 fibroblasts to adipocytes. J Lipid Res 2002; 43:676 - 84; PMID: 11971937
- Chamorro-García R, Kirchner S, Li X, Janesick A, Casey SC, Chow C, Blumberg B. Bisphenol A diglycidyl ether induces adipogenic differentiation of multipotent stromal stem cells through a peroxisome proliferator-activated receptor gamma-independent mechanism. Environ Health Perspect 2012; 120:984 - 9; http://dx.doi.org/10.1289/ehp.1205063; PMID: 22763116
- Sargis RM, Johnson DN, Choudhury RA, Brady MJ. Environmental endocrine disruptors promote adipogenesis in the 3T3-L1 cell line through glucocorticoid receptor activation. Obesity (Silver Spring) 2010; 18:1283 - 8; http://dx.doi.org/10.1038/oby.2009.419; PMID: 19927138
- Wang J, Sun B, Hou M, Pan X, Li X. The environmental obesogen bisphenol A promotes adipogenesis by increasing the amount of 11beta-hydroxysteroid dehydrogenase type 1 in the adipose tissue of children. Int J Obes 2012; 37:999 1005;; PMID: 23090578
- Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 2006; 7:885 - 96; http://dx.doi.org/10.1038/nrm2066; PMID: 17139329
- Abdou HS, Atlas E, Haché RJ. A positive regulatory domain in CCAAT/enhancer binding protein β (C/EBPΒ) is required for the glucocorticoid-mediated displacement of histone deacetylase 1 (HDAC1) from the C/ebpα promoter and maximum adipogenesis. Endocrinology 2013; 154:1454 - 64; http://dx.doi.org/10.1210/en.2012-2061; PMID: 23456364
- Tang QQ, Otto TC, Lane MD. Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci U S A 2004; 101:9607 - 11; http://dx.doi.org/10.1073/pnas.0403100101; PMID: 15210946
- Abdou HS, Atlas E, Haché RJ. Liver-enriched inhibitory protein (LIP) actively inhibits preadipocyte differentiation through histone deacetylase 1 (HDAC1). J Biol Chem 2011; 286:21488 - 99; http://dx.doi.org/10.1074/jbc.M110.211540; PMID: 21521687
- Wiper-Bergeron N, Wu D, Pope L, Schild-Poulter C, Haché RJ. Stimulation of preadipocyte differentiation by steroid through targeting of an HDAC1 complex. EMBO J 2003; 22:2135 - 45; http://dx.doi.org/10.1093/emboj/cdg218; PMID: 12727880
- Vandevyver S, Dejager L, Libert C. On the trail of the glucocorticoid receptor: into the nucleus and back. Traffic 2012; 13:364 - 74; http://dx.doi.org/10.1111/j.1600-0854.2011.01288.x; PMID: 21951602
- Frey FJ, Odermatt A, Frey BM. Glucocorticoid-mediated mineralocorticoid receptor activation and hypertension. Curr Opin Nephrol Hypertens 2004; 13:451 - 8; http://dx.doi.org/10.1097/01.mnh.0000133976.32559.b0; PMID: 15199296
- Nayebosadri A, Christopher L, Ji JY. Bayesian image analysis of dexamethasone and shear stress-induced glucocorticoid receptor intracellular movement. Ann Biomed Eng 2012; 40:1508 - 19; http://dx.doi.org/10.1007/s10439-011-0499-7; PMID: 22227972
- Wu Z, Bucher NL, Farmer SR. Induction of peroxisome proliferator-activated receptor gamma during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPbeta, C/EBPdelta, and glucocorticoids. Mol Cell Biol 1996; 16:4128 - 36; PMID: 8754811
- Hotamisligil GS, Johnson RS, Distel RJ, Ellis R, Papaioannou VE, Spiegelman BM. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science 1996; 274:1377 - 9; http://dx.doi.org/10.1126/science.274.5291.1377; PMID: 8910278
- Wu Z, Rosen ED, Brun R, Hauser S, Adelmant G, Troy AE, McKeon C, Darlington GJ, Spiegelman BM. Cross-regulation of C/EBP alpha and PPAR gamma controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol Cell 1999; 3:151 - 8; http://dx.doi.org/10.1016/S1097-2765(00)80306-8; PMID: 10078198
- Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA). Reprod Toxicol 2007; 24:139 - 77; http://dx.doi.org/10.1016/j.reprotox.2007.07.010; PMID: 17825522
- Matthews JB, Twomey K, Zacharewski TR. In vitro and in vivo interactions of bisphenol A and its metabolite, bisphenol A glucuronide, with estrogen receptors alpha and beta. Chem Res Toxicol 2001; 14:149 - 57; http://dx.doi.org/10.1021/tx0001833; PMID: 11258963
- Hengstler JG, Foth H, Gebel T, Kramer PJ, Lilienblum W, Schweinfurth H, Völkel W, Wollin KM, Gundert-Remy U. Critical evaluation of key evidence on the human health hazards of exposure to bisphenol A. Crit Rev Toxicol 2011; 41:263 - 91; http://dx.doi.org/10.3109/10408444.2011.558487; PMID: 21438738
- Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect 2010; 118:1055 - 70; http://dx.doi.org/10.1289/ehp.0901716; PMID: 20338858
- Tomlinson JJ, Boudreau A, Wu D, Atlas E, Haché RJ. Modulation of early human preadipocyte differentiation by glucocorticoids. Endocrinology 2006; 147:5284 - 93; http://dx.doi.org/10.1210/en.2006-0267; PMID: 16873539
- Carrigan A, Walther RF, Salem HA, Wu D, Atlas E, Lefebvre YA, Haché RJ. An active nuclear retention signal in the glucocorticoid receptor functions as a strong inducer of transcriptional activation. J Biol Chem 2007; 282:10963 - 71; http://dx.doi.org/10.1074/jbc.M602931200; PMID: 17314103
- Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell 1994; 79:1147 - 56; http://dx.doi.org/10.1016/0092-8674(94)90006-X; PMID: 8001151
- Scheja L, Makowski L, Uysal KT, Wiesbrock SM, Shimshek DR, Meyers DS, Morgan M, Parker RA, Hotamisligil GS. Altered insulin secretion associated with reduced lipolytic efficiency in aP2-/- mice. Diabetes 1999; 48:1987 - 94; http://dx.doi.org/10.2337/diabetes.48.10.1987; PMID: 10512363
- Makowski L, Brittingham KC, Reynolds JM, Suttles J, Hotamisligil GS. The fatty acid-binding protein, aP2, coordinates macrophage cholesterol trafficking and inflammatory activity. Macrophage expression of aP2 impacts peroxisome proliferator-activated receptor gamma and IkappaB kinase activities. J Biol Chem 2005; 280:12888 - 95; http://dx.doi.org/10.1074/jbc.M413788200; PMID: 15684432
- Feige JN, Gelman L, Rossi D, Zoete V, Métivier R, Tudor C, Anghel SI, Grosdidier A, Lathion C, Engelborghs Y, et al. The endocrine disruptor monoethyl-hexyl-phthalate is a selective peroxisome proliferator-activated receptor gamma modulator that promotes adipogenesis. J Biol Chem 2007; 282:19152 - 66; http://dx.doi.org/10.1074/jbc.M702724200; PMID: 17468099
- Spooner CJ, Guo X, Johnson PF, Schwartz RC. Differential roles of C/EBP beta regulatory domains in specifying MCP-1 and IL-6 transcription. Mol Immunol 2007; 44:1384 - 92; http://dx.doi.org/10.1016/j.molimm.2006.05.004; PMID: 16784777
- Riu A, Grimaldi M, le Maire A, Bey G, Phillips K, Boulahtouf A, Perdu E, Zalko D, Bourguet W, Balaguer P. Peroxisome proliferator-activated receptor γ is a target for halogenated analogs of bisphenol A. Environ Health Perspect 2011; 119:1227 - 32; http://dx.doi.org/10.1289/ehp.1003328; PMID: 21561829
- Alonso-Magdalena P, Morimoto S, Ripoll C, Fuentes E, Nadal A. The estrogenic effect of bisphenol A disrupts pancreatic beta-cell function in vivo and induces insulin resistance. Environ Health Perspect 2006; 114:106 - 12; http://dx.doi.org/10.1289/ehp.8451; PMID: 16393666
- Ryan KK, Haller AM, Sorrell JE, Woods SC, Jandacek RJ, Seeley RJ. Perinatal exposure to bisphenol-a and the development of metabolic syndrome in CD-1 mice. Endocrinology 2010; 151:2603 - 12; http://dx.doi.org/10.1210/en.2009-1218; PMID: 20351315
- Haché RJ, Tse R, Reich T, Savory JG, Lefebvre YA. Nucleocytoplasmic trafficking of steroid-free glucocorticoid receptor. J Biol Chem 1999; 274:1432 - 9; http://dx.doi.org/10.1074/jbc.274.3.1432; PMID: 9880517
- Miesfeld R, Okret S, Wikström AC, Wrange O, Gustafsson JA, Yamamoto KR. Characterization of a steroid hormone receptor gene and mRNA in wild-type and mutant cells. Nature 1984; 312:779 - 81; http://dx.doi.org/10.1038/312779a0; PMID: 6549049
- Legraverend C, Antonson P, Flodby P, Xanthopoulos KG. High level activity of the mouse CCAAT/enhancer binding protein (C/EBP alpha) gene promoter involves autoregulation and several ubiquitous transcription factors. Nucleic Acids Res 1993; 21:1735 - 42; http://dx.doi.org/10.1093/nar/21.8.1735; PMID: 8493090
- Yeh WC, Cao Z, Classon M, McKnight SL. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev 1995; 9:168 - 81; http://dx.doi.org/10.1101/gad.9.2.168; PMID: 7531665