Abstract
Circulating adipokines are associated with physiological and pathophysiological processes in both obesity and pregnancy. Obesity in pregnancy increases the risk of pregnancy complications and the majority of research uses body mass index (BMI) to assess fatness. Specific fat compartments are associated with obesity-induced health risks yet it is not known how abdominal fat mass in pregnancy is related to circulating adipokines. Plasma leptin, resistin, visfatin, and adiponectin were measured by immunoassay in healthy pregnant women of normal weight (BMI 18.5–24.9; n = 17) and overweight/obese pregnant women (BMI 25.0–40, n = 21) in the third trimester. Total body and abdominal subcutaneous and visceral fat mass were measured at 1–3 weeks postpartum. Overweight/obese women had greater total body fat (t = −6.210, P < 0.001) and abdominal subcutaneous fat (t = −5.072, P < 0.001) than normal-weight women while there was no difference in abdominal visceral fat. Overweight/obese women had higher leptin (66.3 ± 34.2 vs. 35.7 ± 19.3 ng/mL, P < 0.001) compared to normal-weight women. Leptin was associated with total body fat (r = 0.782, P < 0.001) and resistin was associated with abdominal visceral fat (r = 0.452, P = 0.045). No significant correlations were observed between adiponectin or visfatin and any measure of body composition. In pregnant women, resistin has the potential to be a circulating biomarker for visceral fat, an ectopic fat compartment. These observational data may provide insight for the pathophysiological roles of adipokines and the impact of visceral fat in pregnant women.
Introduction
Women of childbearing age in the United States have an obesity rate of 29%.Citation1 Pregravid obesity increases the risk of maternal preeclampsia, gestational diabetes mellitus (GDM), cesarean delivery,Citation2-4 postpartum infection, hemorrhage during delivery,Citation4 and postpartum weight retention.Citation5 Obesity in pregnancy is generally determined by pre-pregnancy body mass index [BMI; body weight (kg) divided by height (m2)]; because abdominal fat is highly inflammatory,Citation6,7 individuals with the same BMI can have large differences in body fat amount and distribution which may be a factor related to pregnancy health risks. Additionally, there is a redistribution from lower-body adiposity toward more central, upper-body adiposity during pregnancy, a shift that is proposed to increase fetal access to stored energy,Citation8 potentially increasing a woman's abdominal adipose stores.
Adipokines such as leptin, adiponectin, resistin, and visfatin may play signaling roles in the low-grade inflammation that occurs in obesity and may contribute to medical pregnancy risks. These adipokines are primarily secreted from adipose tissue and function as important regulators of appetite, glucose homeostasis,Citation9-12 and immune function.Citation13-16 Differences in circulating adipokines in women with pregnancy complications indicate that these adipokines play a role in pathophysiology.Citation17-20 Greater circulating leptin has been shown in pregnant women with greater whole body adiposity measured by either skinfold techniqueCitation21 or body impedance analysis (BIA).Citation22 It was recently reported that plasma adiponectin and body fat (measured by BIA) were independent predictors in overweight/obese pregnant women who developed GDM.Citation23 To date, neither resistin nor visfatin have been shown to correlate with adiposity (whole or abdominal) in pregnant women.
Because there is no information on the relationship of circulating adipokines and abdominal adiposity in regards to pregnancy, the aim of the current study was to define the relationships between leptin, resistin, visfatin, total adiponectin, and high molecular weight adiponectin and body fat compartments (total fat mass, abdominal subcutaneous and visceral fat) in healthy pregnant women. Secondary aims were to assess: 1) if the often-used BMI measurement identified differences in adiposity between BMI groups (healthy weight versus overweight and obese) and 2) if BMI provided adequate sensitivity for assessing adipokines status between groups.
Results
The women in each group were of similar age, parity status, gestational time and weight gain (). Overweight/obese subjects had a 1.7-fold higher total body fat mass compared to normal-weight subjects (t = −6.210, P < 0.001) and 2-fold greater abdominal subcutaneous and visceral fat mass (t = −5.072, P < 0.001) while visceral fat mass did not differ between the 2 BMI groups. Leptin was the only adipokine significantly different between the 2 BMI categories with a ∼2-fold greater concentration in the overweight/obese women vs. normal weight subjects (t = −3.636, P = 0.001, ).
Table 1. Descriptive statistics of subjects by BMI classification
Table 2. Circulating adipokines of subjects by BMI classification
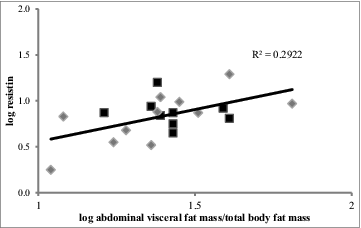
Bivariate correlation showed leptin correlated positively with BMI and total body fat mass (P = 0.001 and P < 0.001, respectively) (). Resistin was the only adipokine positively that correlated with abdominal visceral fat mass relative to total body fat mass (P = 0.045) (). Visceral fat mass/total body fat predicted 29% of variance in resistin regardless of subjects’ pre-pregnancy BMI category (). Neither total and high molecular weight (HMW) adiponectin nor visfatin correlated with total body fat mass or abdominal fat compartments ().
Table 3. Correlation between adipokines and body composition measurements
Figure 1. Relationship of abdominal visceral fat mass/total body fat mass to circulating resistin. Data were log transformed and analyzed by simple linear regression. Black squares (▪) represent subjects with a normal-weight pre-pregnancy BMI (n = 9), gray diamonds (◊) represent subjects with an overweight or obese pre-pregnancy BMI (n = 11). Abdominal visceral fat mass/ total body fat mass predicted 29% of variance in resistin.
Discussion
In this observational study, circulating resistin predicted abdominal visceral adiposity. Resistin is primarily synthesized and secreted by macrophages in the stromal vascular fraction of adipose tissueCitation24 and but also produced by the placentaCitation25 and resistin is generally thought to play a role in insulin resistanceCitation26 and during pregnancy. In fact, resistin has been hypothesized to contribute to the pregnancy-induced insulin resistance needed for fetal development.Citation27 Previous studies found a strong association between resistin and total body fat massCitation28-30 and abdominal subcutaneous fat massCitation30 in healthy non-pregnant adults. However, these studies were performed on non-pregnant women, making comparisons to our study difficult. To our knowledge, our study is the first study to measure both plasma resistin and fat mass compartments in pregnancy.
Three studies have found a relationship between resistin and total body fat mass in non-pregnant populations. Yannakoulia et al.Citation29 and Vozarova de Courten et al.Citation28 measured total body fat mass in adult men and women with BIA and dual-energy X-ray absorptiometry (iDXA), respectively. In addition to an association between resistin and total body fat mass, Won et al.Citation30 found a relationship between resistin and abdominal subcutaneous fat mass, but not visceral fat mass, measured by CT (CT) in adult men and women. Therefore, the increase during resistin that occurs in pregnancy may modulate its relationship to body composition in contrast to observations reported in a non-pregnant population. Additionally, our study quantified MRI images from the entire abdominal area (L2 to S3) whereas single-image protocols localized to L4-L5 are employed by CT prediction of total abdominal adiposity and are only comparable to MRI single image scan prediction models.Citation31
These current findings of a correlation between circulating leptin and BMI and total body fat (measured via iDXA) confirm previous findings of total body fat. Others have reported an association between leptin in the first and third trimester and whole-body fat mass in pregnancy by BIA.Citation21,22 While it has been validated as a reliable estimation for body fat percentage outside of pregnancy,Citation32 BIA has not been validated as an accurate tool for measuring body fat mass during pregnancy. Leptin is secreted primarily from adipocytesCitation24 and in the placenta during pregnancy,Citation33 in vitro perfusion of term placenta releases 98% of synthesized leptin into maternal circulation.Citation34 It is hypothesized that maternal circulation of leptin is important for mobilization of fat deposits to ensure energy availability for the fetus.Citation35 Because measurement of plasma leptin in this study occurred within a narrow gestational timeframe (35–39 weeks), we do not believe differences in placental leptin production were responsible for the differences in leptin concentration seen between normal weight and overweight/obese pregnant women.
It does not appear that abdominal adiposity correlates with circulating adiponectin (total or HMW) or visfatin. O’Sullivan et al.Citation36 found an inverse correlation between total body fat (measured by BIA) and total adiponectin compared to non-pregnant control subjects. To date, no one has reported evidence supporting a relationship between abdominal adiposity and total or HMW adiponectin. Moreover, circulating visfatin does not appear to be associated with adipose tissue levels in pregnancy. Two publications indicate that women with GDM have either higherCitation37 or lower visfatinCitation20 close to delivery. While Telejko et al.Citation20 found no relationship between plasma visfatin to either placental or abdominal subcutaneous or visceral mRNA visfatin expression, Ma et al.Citation37 found higher visfatin placental expression in women with GDM but no change in the 2 adipose tissues.
The strengths of this study include the detailed collection of subject information, the variety of circulating adipokines and body adiposity measurements. This wealth of data allowed us to investigate numerous relationships between subject characteristics, adipokines, and body composition. A limitation was that all of our subjects had generally healthy pregnancies so we could not assess any relationships between adipokines, body fat compartments and pregnancy complications. Another limitation was the number of MRI scans to measure abdominal fat mass compartments as we were only obtained data from 20 subjects. However, this was deemed suitable as these 20 represented both BMI groups (normal weight and overweight/obese). Future studies should include women with pregnancy complications to elucidate the relationship with risky pregnancies to abdominal adiposity and adipokines.
In conclusion, the current study provides significant evidence for resistin was an indicator of abdominal visceral fat mass. Additionally, using iDXA techniques, leptin is validated as a reliable biomarker for whole body fat mass. Finally, while pre-pregnancy BMI was useful in seeing differences in total body fat mass and leptin, there were no differences in visceral fat mass between the BMI groups and it was not sensitive enough to detect any differences in the other 3 adipokines. At least during pregnancy, resistin appears to be a useful clinical marker of visceral fat mass. Visceral fat has been hypothesized to play a central role in obesity-induced pregnancy complications;Citation38-40 however, modification of specific pathways implicated by visceral fat remains largely unexplored. We believe these findings set a benchmark for understanding the relationship between abdominal adiposity and physiologically relevant adipokines in pregnancy.
Materials and Methods
Women from the Kansas City metropolitan area were enrolled between February 2012 and March 2013 as part of the Pregnancy Health Study. Women were eligible if they were English-speaking, 18–45 y old, with a singleton pregnancy, >28 gestational weeks, planning to deliver at the University of Kansas Hospital, and with a pre-pregnancy BMI between 18.5–40 kg/m2 based on self-reported weight and height. Exclusion criteria included diabetes, hypertension, smoking or illegal drug use during pregnancy, chronic medical condition known to influence inflammation status, or fear of enclosed spaces. Gestational weight gain was determined by subtracting self-reported pre-pregnancy weight from the highest weight recorded before delivery in the subjects’ medical record. This study was approved by the University of Kansas Medical Center Human Subjects Committee, and the research protocol and informed consent process was conducted according to the Declaration of Helsinki as amended in October 1996.
Blood samples were obtained from women between 35 and 39 weeks gestation (n = 36) or the morning following birth (n = 2) between 10 a.m. and 4 p.m. to avoid circadian influence on circulating leptin.Citation41 Blood was collected in an EDTA-coated tube and plasma separated from red blood cells and buffy coat (3000 x g, 10 min, 4°C). Women were asked to consume a light meal before their visit to prevent dietary influence on adipokinesCitation42 and body composition measurement. Plasma concentrations of leptin, resistin, and total and HMW adiponectin were determined by ELISA (Alpco Immunoassays, Salem, NH). The lower limits of detection were 0.50, 0.012, and 0.019 ng/mL for leptin, resistin, and adiponectin, respectively, and the inter-assay coefficient of variance (CV) was 1.9%, 2.4%, and 1.7%, respectively. Visfatin was measured by ELISA (Phoenix Pharmaceuticals Inc., Burlingame, CA) with a lower limit of detection of 1.85 ng/mL and an inter-assay CV of 8.7%. All samples were assayed in duplicate.
Total body fat mass was measured between 1–3 weeks postpartum by iDXA (GE Healthcare, Madison, WI) with encore software (Version 13.50) in 31 of the 38 subjects that were available for the measurement. Subjects removed all metal artifacts from their body and were scanned according to standard imaging and positioning protocols. At the same visit, 20 of the 31 underwent an MRI at 3 Tesla (Skyra, Siemens Medical Solutions, Erlangen, Germany)Citation43 for measurement of visceral and subcutaneous abdominal fat mass. To measure abdominal fat mass, we obtained T2-weighted spin echo localizer axial, coronal, and sagittal images while subjects lay on the table in a supine position with arms placed beside their head. Sagittal images were used to identify lumbar vertebrae 2 (L2) and sacral vertebrae 3 (S3) as the landmarks for abdominal fat mass. Blocks of high-resolution axial HASTE TSE T2-weighted images were acquired during 2 16-second breath-holds to minimize breathing motion artifacts. Each acquisition block was comprised of ∼20 axial image slices (5 mm thick, 5 mm gaps). Anatomical locations of the 2 blocks were noted in order to find the consecutive images for analysis and prevent duplication.
Segmentation and quantification of the axial images were performed with ImageJ.Citation44,45 Briefly, a mask was applied to enhance edge definition and adipose and non-adipose tissues were segmented by k-means clustering using custom-designed ImageJ scripts. Subcutaneous and visceral adipose tissues were delineated within each slice and each segmented image was reviewed to correct misclassified pixels caused by blurring of tissue space. Subcutaneous and visceral adipose tissue areas for each image were calculated by summing the highlighted pixels and multiplying by the pixel surface area. The volumes (cm3) were calculated by multiplying the tissue area (cm2) by the slice thickness (5 mm). The adipose tissue volume in the 5.0 mm gap was estimated by averaging the results from the adjacent slices. Finally, the volumes were multiplied by 0.916 g/cm3, the density of adipose tissue, to obtain total mass (g) of the visceral and subcutaneous adipose tissue. All scans were read by a single observer. The intra-rater reliability coefficients of variation for subcutaneous and visceral adipose tissue mass measurements were 0.3% and 5.0%, respectively, based on 2 repeated, blinded, analyses of 5 randomly selected sets of MRI images by a single observer.
Descriptive statistics are presented as mean values ± standard deviation. Linear relationships between body composition and circulating markers were described with Pearson correlation coefficients. Differences between the normal-weight and overweight/obese BMI categories were analyzed with independent t-test. Each abdominal fat compartment (subcutaneous and visceral) was normalized to total body fat mass. Normalizing the abdominal fat compartments to total fat mass reduced variability caused by overall fatness and allowed for assessment of each abdominal adipose tissue compartment to each adipokine. The Shapiro-Wilk test was used to test normality. Outcome variables that were not normally distributed (leptin, resistin, adiponectin, visceral adipose/total body fat mass) were log-transformed prior to analyses. All data were analyzed with IBM SPSS Statistics 17 software (SPSS, Chicago, IL). P-values presented are 2-tailed and statistical significance was set at 0.05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors wish to acknowledge the nursing staffs at our participating clinics and hospital whose support was essential to recruitment and availability of blood samples; Dr. Ping-Chang Lin and Allan Schmitt (Hoglund Brain Imaging Center) for assistance in developing the MRI adipose acquisition and analysis protocol; Kansas Intellectual and Developmental Disabilities Research Center for adipokine analysis; Dr. Eric Gumpricht for manuscript preparation; and our subjects for their time.
Funding
This study is supported by the Sam E. and Mary F. Roberts Foundation, NICHD HD02528, NICHD 5K12HD052027, and NIH S10 RR029577. The Hoglund Brain Imaging Center is supported by a generous gift from Forrest and Sally Hoglund.
References
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA 2006; 295:1549-55; PMID:16595758; http://dx.doi.org/10.1001/jama.295.13.1549
- Weiss JL, Malone FD, Emig D, Ball RH, Nyberg DA, Comstock CH, Saade G, Eddleman K, Carter SM, Craigo SD, et al. Obesity, obstetric complications and cesarean delivery rate–a population-based screening study. Am J Obstet Gynecol 2004; 190:1091-7; PMID:15118648; http://dx.doi.org/10.1016/j.ajog.2003.09.058
- Baeten JM, Bukusi EA, Lambe M. Pregnancy complications and outcomes among overweight and obese nulliparous women. Am J Public Health 2001; 91:436-40; PMID:11236410; http://dx.doi.org/10.2105/AJPH.91.3.436
- Sebire NJ, Jolly M, Harris JP, Wadsworth J, Joffe M, Beard RW, Regan L, Robinson S. Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. Int J Obes Relat Metab Disord 2001; 25:1175-82; PMID:11477502; http://dx.doi.org/10.1038/sj.ijo.0801670
- Vesco KK, Dietz PM, Rizzo J, Stevens VJ, Perrin NA, Bachman DJ, Callaghan WM, Bruce FC, Hornbrook MC. Excessive gestational weight gain and postpartum weight retention among obese women. Obstet Gynecol 2009; 114:1069-75; PMID:20168109; http://dx.doi.org/10.1097/AOG.0b013e3181baeacf
- Lee CM, Huxley RR, Wildman RP, Woodward M. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: a meta-analysis. J Clin Epidemiol 2008; 61:646-53; PMID:18359190; http://dx.doi.org/10.1016/j.jclinepi.2007.08.012
- Koster A, Leitzmann MF, Schatzkin A, Mouw T, Adams KF, van Eijk JT, Hollenbeck AR, Harris TB. Waist circumference and mortality. Am J Epidemiol 2008; 167:1465-75; PMID:18417494; http://dx.doi.org/10.1093/aje/kwn079
- Lassek WD, Gaulin SJ. Changes in body fat distribution in relation to parity in American women: a covert form of maternal depletion. Am J Phys Anthropol 2006; 131:295-302; PMID:16596596; http://dx.doi.org/10.1002/ajpa.20394
- Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 2001; 7:941-6; PMID:11479627; http://dx.doi.org/10.1038/90984
- Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature 1999; 401:73-6; PMID:10485707; http://dx.doi.org/10.1038/43448
- Steppan CM, Lazar MA. Resistin and obesity-associated insulin resistance. Trends Endocrinol Metab 2002; 13:18-23; PMID:11750858; http://dx.doi.org/10.1016/S1043-2760(01)00522-7
- Brown JE, Onyango DJ, Ramanjaneya M, Conner AC, Patel ST, Dunmore SJ, Randeva HS. Visfatin regulates insulin secretion, insulin receptor signalling and mRNA expression of diabetes-related genes in mouse pancreatic beta-cells. J Mol Endocrinol 2010; 44:171-8; PMID:19906834; http://dx.doi.org/10.1677/JME-09-0071
- Fernandez-Riejos P, Najib S, Santos-Alvarez J, Martin-Romero C, Perez-Perez A, Gonzalez-Yanes C, Sanchez-Margalet V. Role of leptin in the activation of immune cells. Mediators Inflamm 2010; 2010:568343; PMID:20368778; http://dx.doi.org/10.1155/2010/568343
- Kusminski CM, da Silva NF, Creely SJ, Fisher FM, Harte AL, Baker AR, Kumar S, McTernan PG. The in vitro effects of resistin on the innate immune signaling pathway in isolated human subcutaneous adipocytes. J Clin Endocrinol Metab 2007; 92:270-6; PMID:17062773; http://dx.doi.org/10.1210/jc.2006-1151
- Moschen AR, Kaser A, Enrich B, Mosheimer B, Theurl M, Niederegger H, Tilg H. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol 2007; 178:1748-58; PMID:17237424; http://dx.doi.org/10.4049/jimmunol.178.3.1748
- Okamoto Y, Folco EJ, Minami M, Wara AK, Feinberg MW, Sukhova GK, Colvin RA, Kihara S, Funahashi T, Luster AD, et al. Adiponectin inhibits the production of CXC receptor 3 chemokine ligands in macrophages and reduces T-lymphocyte recruitment in atherogenesis. Circ Res 2008; 102:218-25; PMID:17991878; http://dx.doi.org/10.1161/CIRCRESAHA.107.164988
- Catalano PM, Hoegh M, Minium J, Huston-Presley L, Bernard S, Kalhan S, Hauguel-De Mouzon S. Adiponectin in human pregnancy: implications for regulation of glucose and lipid metabolism. Diabetologia 2006; 49:1677-85; PMID:16752186; http://dx.doi.org/10.1007/s00125-006-0264-x
- Cortelazzi D, Corbetta S, Ronzoni S, Pelle F, Marconi A, Cozzi V, Cetin I, Cortelazzi R, Beck-Peccoz P, Spada A. Maternal and foetal resistin and adiponectin concentrations in normal and complicated pregnancies. Clin Endocrinol (Oxf) 2007; 66:447-53; PMID:17302882; http://dx.doi.org/10.1111/j.1365-2265.2007.02761.x
- Hendler I, Blackwell SC, Mehta SH, Whitty JE, Russell E, Sorokin Y, Cotton DB. The levels of leptin, adiponectin, and resistin in normal weight, overweight, and obese pregnant women with and without preeclampsia. Am J Obstet Gynecol 2005; 193:979-83; PMID:16157097; http://dx.doi.org/10.1016/j.ajog.2005.06.041
- Telejko B, Kuzmicki M, Zonenberg A, Szamatowicz J, Wawrusiewicz-Kurylonek N, Nikolajuk A, Kretowski A, Gorska M. Visfatin in gestational diabetes: serum level and mRNA expression in fat and placental tissue. Diabetes Res Clin Pract 2009; 84:68-75; PMID:19185944; http://dx.doi.org/10.1016/j.diabres.2008.12.017
- Vega-Sanchez R, Barajas-Vega HA, Rozada G, Espejel-Nunez A, Beltran-Montoya J, Vadillo-Ortega F. Association between adiposity and inflammatory markers in maternal and fetal blood in a group of Mexican pregnant women. Br J Nutr 2010; 104:1735-9; PMID:20650016; http://dx.doi.org/10.1017/S0007114510002825
- Fattah C, Barry S, O'Connor N, Farah N, Stuart B, Turner MJ. Maternal leptin and body composition in the first trimester of pregnancy. Gynecol Endocrinol 2011; 27:263-6; PMID:20528571; http://dx.doi.org/10.3109/09513590.2010.491167
- Ianniello F, Quagliozzi L, Caruso A, Paradisi G. Low adiponectin in overweight/obese women: association with diabetes during pregnancy. Eur Rev Med Pharmacol Sci 2013; 17:3197-205; PMID:24338462
- Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology 2004; 145:2273-82; PMID:14726444; http://dx.doi.org/10.1210/en.2003-1336
- Yura S, Sagawa N, Itoh H, Kakui K, Nuamah MA, Korita D, Takemura M, Fujii S. Resistin is expressed in the human placenta. J Clin Endocrinol Metab 2003; 88:1394-7; PMID:12629135; http://dx.doi.org/10.1210/jc.2002-011926
- Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature 2001; 409:307-12; PMID:11201732; http://dx.doi.org/10.1038/35053000
- Nien JK, Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Gotsch F, Pineles BL, Friel LA, Espinoza J, Goncalves L, et al. Resistin: a hormone which induces insulin resistance is increased in normal pregnancy. J Perinat Med 2007; 35:513-21; PMID:17919114
- Vozarova de Courten B, Degawa-Yamauchi M, Considine RV, Tataranni PA. High serum resistin is associated with an increase in adiposity but not a worsening of insulin resistance in Pima Indians. Diabetes 2004; 53:1279-84; PMID:15111497; http://dx.doi.org/10.2337/diabetes.53.5.1279
- Yannakoulia M, Yiannakouris N, Bluher S, Matalas AL, Klimis-Zacas D, Mantzoros CS. Body fat mass and macronutrient intake in relation to circulating soluble leptin receptor, free leptin index, adiponectin, and resistin concentrations in healthy humans. J Clin Endocrinol Metab 2003; 88:1730-6; PMID:12679465; http://dx.doi.org/10.1210/jc.2002-021604
- Won JC, Park CY, Lee WY, Lee ES, Oh SW, Park SW. Association of plasma levels of resistin with subcutaneous fat mass and markers of inflammation but not with metabolic determinants or insulin resistance. J Korean Med Sci 2009; 24:695-700; PMID:19654955; http://dx.doi.org/10.3346/jkms.2009.24.4.695
- Lee S, Janssen I, Ross R. Interindividual variation in abdominal subcutaneous and visceral adipose tissue: influence of measurement site. J Appl Physiol 2004; 97:948-54; PMID:15121737; http://dx.doi.org/10.1152/japplphysiol.01200.2003
- Sun G, French CR, Martin GR, Younghusband B, Green RC, Xie YG, Mathews M, Barron JR, Fitzpatrick DG, Gulliver W, et al. Comparison of multifrequency bioelectrical impedance analysis with dual-energy X-ray absorptiometry for assessment of percentage body fat in a large, healthy population. Am J Clin Nutr 2005; 81:74-8; PMID:15640463
- Henson MC, Swan KF, O'Neil JS. Expression of placental leptin and leptin receptor transcripts in early pregnancy and at term. Obstet Gynecol 1998; 92:1020-8; PMID:9840570; http://dx.doi.org/10.1016/S0029-7844(98)00299-3
- Linnemann K, Malek A, Sager R, Blum WF, Schneider H, Fusch C. Leptin production and release in the dually in vitro perfused human placenta. J Clin Endocrinol Metab 2000; 85:4298-301; PMID:11095471
- D'Ippolito S, Tersigni C, Scambia G, Di Simone N. Adipokines, an adipose tissue and placental product with biological functions during pregnancy. Biofactors 2012; 38:14-23; PMID:22287297; http://dx.doi.org/10.1002/biof.201
- O'Sullivan AJ, Kriketos AD, Martin A, Brown MA. Serum adiponectin levels in normal and hypertensive pregnancy. Hypertens Pregnancy 2006; 25:193-203; PMID:17065040; http://dx.doi.org/10.1080/10641950600912976
- Ma Y, Cheng Y, Wang J, Cheng H, Zhou S, Li X. The changes of visfatin in serum and its expression in fat and placental tissue in pregnant women with gestational diabetes. Diabetes Res Clin Pract 2010; 90:60-5; PMID:20621376; http://dx.doi.org/10.1016/j.diabres.2010.06.010
- Denison FC, Roberts KA, Barr SM, Norman JE. Obesity, pregnancy, inflammation, and vascular function. Reproduction 2010; 140:373-85; PMID:20215337; http://dx.doi.org/10.1530/REP-10-0074
- Huda SS, Brodie LE, Sattar N. Obesity in pregnancy: prevalence and metabolic consequences. Semin Fetal Neonatal Med 2010; 15:70-6; PMID:19896913; http://dx.doi.org/10.1016/j.siny.2009.09.006
- King JC. Maternal obesity, metabolism, and pregnancy outcomes. Annu Rev Nutr 2006; 26:271-91; PMID:16704347; http://dx.doi.org/10.1146/annurev.nutr.24.012003.132249
- Sinha MK, Ohannesian JP, Heiman ML, Kriauciunas A, Stephens TW, Magosin S, Marco C, Caro JF. Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. J Clin Invest 1996; 97:1344-7; PMID:8636448; http://dx.doi.org/10.1172/JCI118551
- Korbonits M, Trainer PJ, Little JA, Edwards R, Kopelman PG, Besser GM, Svec F, Grossman AB. Leptin levels do not change acutely with food administration in normal or obese subjects, but are negatively correlated with pituitary-adrenal activity. Clin Endocrinol (Oxf) 1997; 46:751-7; PMID:9274707; http://dx.doi.org/10.1046/j.1365-2265.1997.1820979.x
- Ross R, Leger L, Morris D, de Guise J, Guardo R. Quantification of adipose tissue by MRI: relationship with anthropometric variables. J Appl Physiol 1992; 72:787-95; PMID:1559959
- Rasband W. Image J. Bethesda, Maryland: U.S. National Institutes of Health 1997-2014; retrieved from http://imagej.nih.gov/ij
- McNeill DR, Lin PC, Miller MG, Pistell PJ, de Souza-Pinto NC, Fishbein KW, Spencer RG, Liu Y, Pettan-Brewer C, Ladiges WC, et al. XRCC1 haploinsufficiency in mice has little effect on aging, but adversely modifies exposure-dependent susceptibility. Nucleic Acids Res 2011; 39:7992-8004; PMID:21737425; http://dx.doi.org/10.1093/nar/gkr280
