Abstract
The formation of new adipocytes from precursor cells is a crucial aspect of normal adipose tissue function. During the adipogenic process, adipocytes differentiated from mesenchymal stem cells give rise to two main types of fat: white adipose tissue (WAT) characterized by the presence of adipocytes containing large unilocular lipid droplets, and brown adipose tissue (BAT) composed by multilocular brown adipocytes packed with mitochondria. WAT is not only important for energy storage but also as an endocrine organ regulating whole body homeostasis by secreting adipokines and other mediators, which directly impact metabolic functions in obesity. By contrast, BAT is specialized in dissipating energy in form of heat and has salutary effects in combating obesity and associated disorders. Unfortunately, WAT is the predominant fat type, whereas BAT is scarce and located in discrete pockets in adult humans. Luckily, another type of brown adipocytes, called beige or brite (brown-in-white) adipocytes, with similar functions to those of “classical” brown adipocytes has recently been identified in WAT. In this review, a close look is given into the role of bioactive lipid mediators in the regulation of adipogenesis, with a special emphasis on the role of the microsomal prostaglandin E (PGE) synthase-1, a terminal enzyme in PGE2 biosynthesis, as a key regulator of white-to-brown adipogenesis in WAT.
Abbreviations::
- 15d-PGJ2, 15-deoxy-Δ12,14-prostaglandin J2
- 20-HETE, 20-hydroxy eicosatetraenoic acid
- Akt, protein kinase B
- ATP, adenosine triphosphate
- ATGL, adipose triglyceride lipase
- BAT, brown adipose tissue
- BMPs, bone morphogenic proteins
- cAMP, cyclic adenosine 3′ 5′-monophosphate
- C/EBP, CCAAT enhancer binding protein
- CIDEA, cell death-inducing DFFA-like effector a
- COX, cyclooxygenase
- FLAP, 5-lipoxygenase activating protein
- IL, interleukin
- LOX, lipoxygenase
- LT, leukotriene
- MCP-1, monocyte chemotactic protein-1
- mPGES-1, microsomal prostaglandin E (PGE) synthase-1
- Myf-5, mesenchymal myogenic factor-5
- PGE2, prostaglandin E2
- PAI-1, plasminogen activator inhibitor-1
- PPARγ, peroxisome proliferator-activated receptor γ
- PGC-1α, PPARγ co-activator-1 α
- PRDM16, PR domain containing 16
- TG, triglyceride
- TGFβ, transforming growth factor β
- UCP1, uncoupling protein 1
- WAT, white adipose tissue
- Wnt5b, wingless-type MMTV integration site family, member 5B
- Zfp423, zinc-finger protein 423
White versus Brown Adipose Tissue
White adipose tissue (WAT) adipocytes are nucleated cells comprising a characteristic unilocular lipid droplet mainly composed of triglycerides (TGs) and cholesterol esters, which occupies most of the cell, and a thin rim of cytoplasm displaced to the periphery.Citation1 The major function of WAT is the formation (adipogenesis) and storage of lipids (i.e., fatty acids) in the form of TGs within periods of less energy expenditure.Citation1 Indeed, during times in which energy intake is higher than the metabolic demand, adipocytes would expand nearly 1000-fold in volume and 10-fold in diameter in order to store the excess of fuel as TGs.Citation1 In contrast, in periods of food restriction or in periods demanding more energy expenditure, adipose tissue serves, via lipolysis, as the major source of fatty acids. Under starving conditions, lipolysis is an essential mechanism whereby rate-limiting enzymes such as hormone-sensitive lipase, adipose triglyceride lipase (ATGL) and monoglyceride lipase catalyze hydrolysis of TGs to release free fatty acids (FFA) into the circulation.Citation1 Circulating FFA are subsequently taken up via fatty acid transporters present in metabolically active and insulin-sensitive tissues (primarily skeletal muscle and liver), which then use these lipids as energy substrates for generation of adenosine triphosphate (ATP) high-energy bonds for metabolic functions trough oxidative phosphorylation.Citation2 Extensive research has been conducted on this topic, especially on mechanisms regulating lipolysis, which is a highly regulated process under the control of the sympathetic nervous system, hormones and other paracrine/autocrine factors.Citation1,2 Most of these studies have focused on the pathogenic consequences of the disruption of lipolysis and lipogenesis equilibrium and their role on the onset of type 2 diabetes, insulin resistance, and non-alcoholic fatty liver disease.
Although WAT has classically been considered a mere storage of fat and energy and with a limited role in thermal insulation, this tissue is now generally recognized as an important endocrine organ that secretes a number of hormones and signaling factors, collectively known as adipokines.Citation3 Adipokines released by WAT exert diverse biological functions such as regulation of appetite and satiety, glucose and lipid metabolism, blood pressure homeostasis, inflammation and immune functions.Citation3-5 More than 50 different molecular entities secreted from WAT have been described, including cytokines and related proteins (leptin, tumor necrosis factor [TNF] α, interleukin [IL]-6, IL-10, and monocyte chemotactic protein-1 [MCP-1]), proteins of the fibrinolytic cascade (plasminogen activator inhibitor-1 [PAI-1]), complement-related proteins (visfatin and adiponectin), and other biologically active peptides such as resistin and chemerin.Citation3-5 Detailed information on the role of these adipokines in whole body homeostasis can be found elsewhere.Citation3-5
In addition to WAT, another type of adipose tissue exists in mammals: the brown adipose tissue (BAT). Compared with WAT, BAT contains smaller adipocytes and uses the chemical energy in lipids to produce heat through non-shivering thermogenesis.Citation6,7 BAT adipocytes are brown rather than white because they are packed with mitochrondia. Moreover BAT adipocytes contain uncoupling protein 1 (UCP1), an integral membrane protein that acts as a proton channel diverting the respiratory chain from ATP synthesis to the generation of heat.Citation6,7 In other words, the uncoupling of the oxidative phosphorylation in BAT adipocyte mitochrondia results in the combustion of the excess of lipids and the dissipation of heat by its distribution to the rest of the body through the circulation.Citation6,7 According to these unique properties, BAT is in the focus of scientific interest for the search of novel therapies aimed at combating metabolic diseases and complications associated with obesity, such as type 2 diabetes.Citation6,7 Unfortunately, BAT is difficult to find in adult humans, since most of BAT pads existing within the posterior neck in neonatal humans to provide cold adaptive thermogenesis for newborns are lost soon after birth.Citation6,7 Nevertheless, recent studies using non-invasive imaging technologies such as fluorolabeled 2-deoxy-glucose positron emission tomography (18FDG-PET) scanning and MRI scanning have clearly demonstrated that significant amounts of BAT deposits are still active in adult humans.Citation8
In addition to “classical” brown adipocytes found in BAT, a second type of brown adipocytes termed “beige” or “brite” cells has been identified in WAT. Beige cells are defined by their multilocular lipid droplet morphology, high mitochondrial content and the expression of a core set of brown fat-specific genes (UCP1, cell death-inducing DFFA-like effector A [CIDEA] and peroxisome proliferator activated receptor gamma (PPARγ) coactivator 1 α [PGC-1α]).Citation9 In contrast to “classical” brown adipocytes, beige cells do not derive from pluripotent mesenchymal myogenic factor-5 (Myf-5)-positive cells (see below).Citation10 In addition, these two cell types are differentially regulated since a number of quantitative trait loci are associated with the induced development of beige adipocytes but not “classical” brown adipocytes.Citation11 Furthermore, while “classical” brown adipocytes express high levels of UCP1 and other thermogenic genes under basal conditions, beige cells only express these genes in response to agonists of the β-adrenergic receptors and PPARγ.Citation12 The existence of this separate type of brown adipocytes in WAT has been recently reinforced by the cloning of beige-cell lines from mouse inguinal WAT.Citation13 Unexpectedly, recent studies have demonstrated that a number of beige cell-selective genes are abundantly expressed in human BAT depots, suggesting that human BAT may also be composed of beige/brite cells.Citation9,13 Together, the engagement of beige/brite adipocytes and the induction of a thermogenic program in WAT depots are relevant in terms of energy homestasis because they ultimately exert protection against obesity and obesity-related co-morbidities by wasting the surplus of energy through increased heat production. In this regard, increases in number of beige cells in WAT are closely associated with a protection against diet-induced obesity and metabolic diseases.Citation9
Adipogenesis
Adipocytes, like other mesenchymal cells, are generally described to derive from the mesodermal layer of the embryo. White adipocytes are believed to derive from the lateral plate mesoderm, whereas brown adipocytes would originate from paraxial mesoderm.Citation14 The potential of paraxial mesoderm to differentiate into BAT was initially suggested by Loncar et al.,Citation15 who performed pioneer transplantation of mesoderm from a 9-day-old rat embryo under the renal capsule of a recipient, resulting in the exclusive generation of BAT. The mesodermal origin of interscapular BAT was later confirmed through an in vivo lineage tracing strategy, in which myogenin-expressing dermomyotome, a paraxial mesoderm-derived structure, gave rise to dermis and muscle as expected, but also to BAT.Citation16 The expression of Myf-5 is an established marker used to distinguish white (Myf-5-negative) from brown (Myf-5-positive) adipocytes.Citation7 Similar to white adipocytes, beige/brite cells are apparently derived from cell precursors originated in the lateral plate mesoderm, although the origin of these cells is still controversial.Citation7 Approximately 300 genes were identified by microarray analysis to be differentially expressed in undifferentiated primary brown as compared with white adipocyte progenitors.Citation17 Subsequent studies using muscle-selective Myf-5-Cre knock-in mice confirmed that skeletal muscle and “classical” brown adipocytes, but not white adipocytes, arise from common Myf-5-expressing progenitors.Citation18
Adipogenesis takes place in two different phases: determination of preadipocytes from multipotent stem cells and terminal differentiation of preadipocytes into mature adipocytes. The first phase involves the commitment of pluripotent stem cells to the adipocyte lineage (preadipocyte), which cannot be distinguished morphologically from its cell precursors but has lost the potential to differentiate into osteoblasts, myocytes, and chondrocytes.Citation19 Several genes have been involved in this process, including the wingless-type MMTV integration site family, member 5B (Wnt5b), transforming growth factor (TGF) β, and bone morphogenic proteins (BMPs). The β-catenin-independent Wnt5b ligand has been shown to promote adipogenesis by targeting alternative cell surface receptors and inhibiting β-catenin nuclear translocation.Citation20 In turn, TGF-β has been shown to exert either pro- or anti-adipogenic actions through stimulation of the transcription factor mothers against decapentaplegic homolog (also known as SMAD) in different in vitro and ex vivo models.Citation21 Finally, several BMPs also promote preadipocyte commitment via SMAD/p38 signaling.Citation22 Specifically, BMP2 and BMP4 promote preadipocyte determination, whereas BMP7 promotes brown adipogenesis.Citation23 The zinc-finger protein 423 (Zfp423) also induces adipose lineage commitment in part through amplification of the BMP signaling pathway, an effect that depends on its SMAD-binding capacity.Citation24
In the second phase (i.e., terminal differentiation), preadipocytes take on the characteristics of mature adipocytes. In the case of mature white adipocytes, cells acquire the machinery that is necessary for lipid transport and synthesis, insulin sensitivity and the secretion of adipocyte-specific proteins.Citation25 Several factors have been described to be involved in the terminal differentiation of preadipocytes, once these cells have committed to the adipogenic lineage. The most relevant is PPARγ, which is defined as the “master regulator” of fat cell formation, since PPARγ is not only necessary but also sufficient for the adipogenic process. Indeed, most of the transcription factors identified as promoters or inhibitors of adipogenesis (i.e., PR domain containing 16 [PRDM16], CCAAT enhancer binding protein [C/EBP] α and β, and PGC-1α) exert their actions by either inducing or repressing PPARγ.Citation22,25 For example, PRDM16 is a BAT-specific transcription factor that interacts with PGC-1α (a PPARγ co-activator) and C/EBPβ to trigger a brown adipocyte differentiation program.Citation18 On the other hand, C/EBPα induces PPARγ in a positive feedback loop, thereby maintaining the differentiated cell state of adipocytes.Citation22 Finally, PGC-1α is a PPARγ coactivator that regulates the expression of mitochrondial genes involved in adaptive thermogenesis, like UCP1, in brown adipocytes.Citation22
Local Production of Bioactive Lipid Mediators in WAT
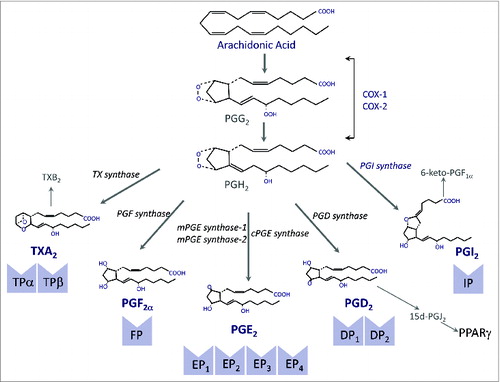
The ability of WAT to generate bioactive lipid mediators was first described in the late 1960s when Shaw and Ramwell identified a group of hydroxyl C20 carboxylic acids known as prostaglandins (PGs) derived from the oxygenation of the polyunsaturated fatty acid arachidonic acid in rat epipidymal fat pads.Citation26 A schematic diagram of the PG biosynthetic pathway is given in . Among the different cyclooxygenase (COX)-derived products in WAT, PGE2 was recognized as one of the most abundant PGs.Citation26 This finding was consistent with previous studies pointing to PGE2 as a negative regulator of hormone-stimulated lypolysis.Citation27 Along these lines, pre-incubation of adipocytes with COX inhibitors was reported to enhance lipolysis.Citation28 Given that the lipolytic actions of catecholamines are mediated by cyclic adenosine 3′,5′-monophosphate (cAMP), which in turn activates TG lipase, the anti-lipolytic actions of PGE2 are likely associated with the modulation of adipocyte cAMP levels.Citation29 In addition to PGE2, the COX-derived product 15-deoxy-ΔCitation12,14-prostaglandin J2 (15d-PGJ2) has been postulated to play a physiologically relevant role in adipose tissue. 15d-PGJ2 is a cyclopentenone metabolite produced by dehydration of PGD2.Citation30 The predominant enzymatic source of 15d-PGJ2 formation in vivo is COX-2 and unlike other PGs, no specific membrane receptor has been identified for this lipid mediator.Citation30 Instead, 15d-PGJ2 exerts its anti-inflammatory and adipogenic actions through binding and activation of the nuclear receptor PPARγ ().Citation31
Figure 1. The cyclooxygenase (COX) pathway. COX exists in two different isoforms (COX-1 and COX-2) and oxygenates arachidonic acid to form prostaglandin (PG) G2 that is further reduced to PGH2. PGH2 is a highly unstable endoperoxide that is rapidly converted by specific synthases to PGs of the E, F and D series and also to PGI2 (prostacyclin) and thromboxane (TX) A2. Both PGI2 and TXA2 have very short half-lifes and are rapidly hydrolyzed to the inactive compounds 6-keto-PGF1α and TXB2, respectively. PGD2 undergoes nonenzymatic dehydration, losing water to form the cyclopentenone 15-deoxy-Δ12-14-PGJ2 (15d-PGJ2). The biological effects of PGs are mediated by ten different types and subtypes of receptors, which belong to the G protein-coupled rhodopsin-type receptor superfamily of seven transmembrane domains. Four of the receptor subtypes bind PGE2 (EP1, EP2, EP3 and EP4), two bind PGD2 (DP1 and DP2), two bind TXA2 (TPα and TPβ) and the rest are single receptors for PGF2α and PGI2 (FP and IP, respectively). 15d-PGJ2 is a natural ligand of PPARγ.
Apart from COX products, WAT also has the ability to produce and release lipoxygenase (LOX) products, especially leukotriene (LT) B4, which apparently is the predominant LOX metabolite in this tissue.Citation32 In this regard, WAT expresses all enzymes necessary for the formation of 5-LOX products (5-LOX, 5-LOX activating protein [FLAP], LTA4 hydrolase, and LTC4 synthase).Citation32 LTB4 has been identified as an inflammatory factor in WAT and FLAP overexpression and excessive generation of 5-LOX products are common findings in WAT of obese patients and animals with insulin resistance.Citation32,33 Moreover, a direct relationship between LTB4 and enhanced release of inflammatory adipokines (i.e., MCP-1 and IL-6) has been reported in obese WAT.Citation32 Consistent with this finding, a selective FLAP has been shown to alleviate adipose tissue inflammation and insulin resistance in obesity.Citation32 Neither a role in adipogenesis nor a role in WAT lipolysis has been demonstrated for LTB4.Citation34
In addition to heightened production of pro-inflammatory COX and LOX-derived lipid mediators, obese WAT also has the capacity to generate anti-inflammatory lipid mediators. Indeed, WAT expresses all enzymes necessary for the biosynthesis of resolvins, protectins, and maresins derived from omega-3 polyunsaturated fatty acids, as well as all receptors necessary for their signaling (reviewed in Spite et al. 2014).Citation35 Moreover, a deficit in the levels of these endogenous anti-inflammatory and pro-resolving lipid mediators has been characterized by means of LC-MS/MS-based metabolo-lipidomic analyses in inflamed visceral and subcutaneous fat compartments from ob/ob obese mice and db/db obese/diabetic mice.Citation35 In humans, a remarkable deficit in these mediators has been reported in subcutaneous fat from patients with peripheral vascular disease, in whom the inflammatory status of WAT is profoundly exacerbated.Citation35 In patients with vascular disease, unique signature profiles and a heterogeneous capacity to generate pro-resolving lipid mediators were identified among WAT from different anatomic locations, being the perivascular fat the depot with higher biosynthetic capacity. Consistent with these observations, administration of exogenous pro-resolving mediators successfully rescued the impaired resolution capacity of obese WAT, enhanced the expression and secretion of adiponectin in parallel with decreased secretion of pro-inflammatory adipokines/cytokines including leptin, TNFα, IL-6, and IL-1β, improved glucose tolerance, decreased fasting blood glucose, and increased insulin-stimulated Akt (also known as protein kinase B) phosphorylation in this tissue.Citation35 In human monocyte-adipocyte assays, these pro-resolving mediators reduced MCP-1 and LTB4-stimulated monocyte adhesion to adipocytes as well as monocyte transadipose migration, which are likely events in the progression of inflamed adipose tissue.Citation35 Interestingly, proresolving mediators skewed adipose tissue macrophages toward an M2 phenotype, which is anti-inflammatory in nature.Citation35 Collectively, these findings are consistent with the notion that unresolved chronic “low grade” inflammation in obese adipose tissue is the result of an inappropriate resolution capacity allowing the inflammatory response to proceed uncontrolled. At present, a role for these omega-3-derived anti-inflammatory lipid mediators in WAT adipogenesis and lipolysis still remains elusive.
Signaling Pathways Involved in the Biological Actions of PGE2 and Other Lipid Mediators
PGE2 binds four different receptor subtypes designated EP1, EP2, EP3, and EP4 ().Citation36 These receptor subtypes differ in their signal transduction pathways: EP1 is coupled to the mobilization of intracellular Ca2+ stores; EP2 and EP4 are coupled to the stimulation of adenylate cyclase whereas EP3 is coupled to the inhibition of adenylate cyclase.Citation36 Early work on adipose tissue demonstrated that PGE2 increases oxygen consumption in rat brown adipose tissue via EP1 receptor.Citation37 More recent work has demonstrated that PGE2 may suppress 3T3-L1 adipocyte differentiation by binding to EP4 and eliciting an increase in intracellular cAMP levels in preadipocytes.Citation38 On the other hand, addition of the PGD2 metabolite 15d-PGJ2, which is a natural PPARγ ligand, to human adipocytes inhibits the secretion of pro-inflammatory adipokines and more importantly stimulates adipogenesis.Citation39 15d-PGJ2 also exerts proadipogenic actions in fibroblasts, although in this case lymphocytes are the source of this cyclopentenone PG.Citation40 Surprisingly, impaired adipogenic program has been identified in 3T3-L1 cells with stable transfection of PGD synthase and appreciably higher levels of endogenous PGD2-derived metabolites, suggesting a complex regulatory interaction between PPARγ and proadipogenic lipid mediators.Citation41 Finally, 5-LOX-derived LTs signal through two receptors for LTB4 (BLT-1 and BLT-2) and two receptors for cys-LTs (CysLT1 and CysLT2).Citation32 Of interest, mice deficient for the LTB4 receptor BLT-1 show reduced monocyte recruitment to hypertrophied adipose tissue and decreased adipose tissue inflammation and insulin resistance.Citation42
Microsomal PGE Synthase-1 (mPGES-1) is Involved in a White-to-Brown Transition of the Adipogenic Program
A coordinate interaction between mPGES-1 and PPARγ in controlling the process of preadipocyte differentiation in WAT has recently been identified.Citation34 mPGES-1 is an inducible enzyme that cooperates with COX-2, the first upstream enzyme of the PG biosynthetic cascade, in the biosynthesis of PGE2.Citation43 Several studies have previously linked the COX pathway and PGE2 to the adipogenic program, although their results have yielded controversial views. For example, preadipocytes stably transfected with either COX-1 or COX-2 were shown to have a lower PPARγ expression and to exhibit a suppression of the adipogenic program.Citation44 This finding is consistent with the observation that mice genetically deficient for mPGES-1 show basal elevations in PPARγ expression and transcriptional activity of this nuclear receptor.Citation45 In contrast, PPARγ appears to be downregulated in adipose tissue from COX-2 deficient mice, which show an attenuation in adipocyte differentiation.Citation46 Also, Kim and collaborators recently described that a COX-2 dependent metabolism of 20-hydroxy-eicosatetraenoic acid (20-HETE) induces PPARγ and the adipogenic program in mesenchymal stem cell-derived adipocytes.Citation47 On the other hand, COX-2-deficient mice provided the first proof of concept that UCP1 expression in WAT is dependent on COX activity.Citation48 A recent study by Vegiopoulos and collaborators has demonstrated that transgenic mice overexpressing COX-2 did not exhibit changes in PPARγ expression in adipose tissues.Citation49 An interesting finding of this study was that overexpression of COX-2 was associated with de novo recruitment of brown adipocytes in WAT, suggesting that PGs may be involved in the browning of WAT.Citation49 However, the study by Vegiopoulos and collaborators poses the limitation that in addition to PGE2, COX-2 activity gives rise to other PGs, including PGI2, PGF2α, and PGD2, and therefore, this study left unanswered the relative role of each individual PG in the development of brown adipocytes in WAT. Moreover, this study did not provide a plausible hypothesis whether WAT beige cells come from progenitor cells (preadipocytes) pushed to develop into beige/brown adipocytes by PGs or whether PGs induce the direct conversion of differentiated white adipocytes into beige cells.
In our study, we obviated these limitations by focusing our interest on mPGES-1, a COX-2 down-stream terminal synthase responsible for the biosynthesis of PGE2, and by exploring the direct actions of this lipid mediator on preadipocytes isolated from WAT.Citation34 Using this approach, we were able to gather evidence that mice lacking PPARγ specifically in the adipose tissue are resistant to gain body weight and to increase WAT volume by mechanisms linked to PGE2 formation.Citation34 Indeed, the results from our study support a coordinated negative regulation between PPARγ and PGE2. On one hand, mice deficient in PPARγ showed increased expression of COX-2 and mPGES-1 and augmented PGE2 levels. On the other hand, exogenous PGE2 was able to suppress PPARγ expression whereas opposite effects were seen after inhibition of endogenous PGE2 biosynthesis in adipose tissue.Citation34 Moreover, this mPGES-1-derived product was able to divert preadipocyte differentiation in WAT to beige/brite mature adipocytes accompanied by upregulation of UCP1. A proof of concept of the role of mPGES-1 was obtained by inhibiting either the expression or the activity of this terminal enzyme. In particular, the addition of a selective pharmacological mPGES-1 inhibitor as well as a siRNA directed against mPGES-1 to preadipocytes resulted in the reduction of browning markers (i.e., UCP1, CIDEA, and PGC-1α) and browning determination factors (i.e., PRDM16).Citation34 A schematic diagram of the hypothetical coordinated functional regulation between mPGES-1 and PPARγ in beige/brite adipogenesis is shown in .
Figure 2. Schematic diagram of the proposed coordinated functional regulation of microsomal prostaglandin E (PGE) synthase-1 (mPGES-1) and peroxisome proliferator-activated receptor γ (PPARγ) in beige/brite adipogenesis in white adipose tissue (WAT). In WAT preadipocytes, mPGES-1 cooperates with cyclooxygenase-2 (COX-2) in the biosynthesis and release of prostaglandin (PG) E2 from arachidonic acid. By binding to its receptors (presumably PGE2 receptor EP4 subtype), PGE2 is able to downregulate PPARγ expression, which in turn suppresses COX-2 and mPGES-1 expression in an autocrine fashion. Furthermore, the interaction between PGE2 and PPARγ has the ability to induce brown adipogenic genes such as uncoupling protein 1 (UCP1), cell death-inducing DFFA-like effector a (CIDEA), PPARγ co-activator-1α (PGC-1α), and PR domain containing 16 (PRDM16), which divert pre-adipocyte differentiation into beige/brite adipocytes instead of white adipocytes.
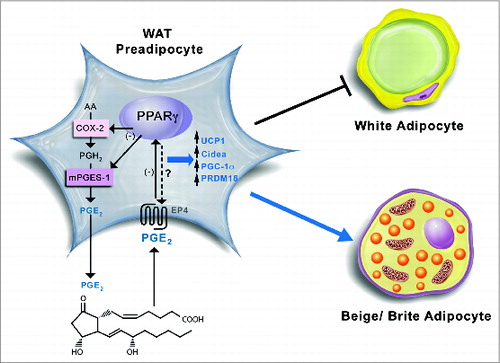
The mechanisms by which the COX-2/mPGES-1/PGE2 axis and the nuclear receptor PPARγ interact during the process of adipogenesis are not completely delineated but might be related to the ability of PGE2 to increase intracellular cAMP, which is a well-known mediator of the induction of “brown fat-like” cells residing in the WAT.Citation50 Another mechanism potentially implicated is the stabilization of several dominant transcriptional regulators of white-to-brown adipocyte development and function, including PRDM16.Citation51 In fact, a reduction in PRDM16 was observed in adipocytes incubated with an inhibitor of mPGES-1 activity or transfected with a siRNA that induce gene silencing of this terminal PG synthase.Citation34 Apparently, this process requires the integrity of both systems because PGE2 was not able to inhibit white adipocyte differentiation in the absence of a PPARγ agonist.Citation34 Moreover, PGE2 was able to directly induce the browning of WAT (i.e., upregulating UCP1) in wild-type mice but not in mice lacking PPARγ specifically in adipocytes.Citation34 Together, these findings are relevant in terms of energy homestasis because the engagement of beige/brite adipocytes and the induction of a thermogenic program in WAT depots are able to waste the surplus of energy through increased heat production, which ultimately exerts protection against obesity and obesity-related co-morbidities.
Summary and Future Perspectives
A wealth of new evidence supports the concept that browning of WAT has a therapeutic potential in fighting the metabolic complications associated with obesity. It is interesting to mention that even small amounts of active brown adipose tissue lead to an enhanced energy expenditure and to the promotion of weight loss accompanied by reductions in the incidence of obesity comorbidities such as type 2 diabetes and cardiovascular disease.Citation52 A plethora of novel factors carrying capacity to induce brown adipocyte differentiation has been recently identified, although the understanding of the mechanisms underlying their actions is still elusive. The observation that exposure of preadipocytes from WAT origin to the bioactive lipid mediator PGE2 results in a browning effect during the adipocyte differentiation process adds value to the fact that COX-2-activity induces de novo recruitment of brown adipocytes in WAT. In summation, these studies point into the COX-2/mPGES-1/PGE2 axis as a key regulator of the browning process in WAT. Considering that in addition to promote the browning and heat dissipating function in WAT, the COX-2/mPGES-1/PGE2 pathway also has a direct responsibility in mounting inflammatory responses, further studies are needed to fully integrate the duality of PGE2 actions in obese conditions. This is of special interest in the context of the “low-grade” inflammatory state present in obese WAT, which is directly linked to the development of the obesity-associated metabolic complications. Further studies are also needed to understand whether the approach of inducing the browning of WAT by bioactive lipid mediators described in animal models is also efficacious in humans. Finally, an important aspect that also needs further investigation is whether PGE2-induced activation of beige/brite adipocytes takes place not only in lean individuals but also in WAT from obese patients.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Our laboratory is supported by a grant from the Ministerio de Ciencia e Innovación (MICINN) (SAF12/32789) and is a Consolidated Research Group recognized by the Generalitat de Catalunya (2014SGR428). CIBERehd is funded by the Instituto de Salud Carlos III. V.G.-A. has a fellowship from MICINN (BES-2010-034193).
References
- Adipose Tissue Biology, ed. Michael E. Symonds. Springer, New York, NY, 2012.
- Wang P, Mariman E, Renes J, Keijer J. The secretory function of adipocytes in the physiology of white adipose tissue. J Cell Physiol 2008; 216:3-13; PMID:18264975; http://dx.doi.org/10.1002/jcp.21386
- Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol 2011; 11:85-97; PMID:21252989; http://dx.doi.org/10.1038/nri2921
- González-Périz A, Clària J. Resolution of adipose tissue inflammation. ScientificWorldJournal 2010; 10:832-56; PMID:20454765; http://dx.doi.org/10.1100/tsw.2010.77
- Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol 2011; 29:415-45; PMID:21219177; http://dx.doi.org/10.1146/annurev-immunol-031210-101322
- Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med 2013; 19:1252-63; PMID:24100998; http://dx.doi.org/10.1038/nm.3361
- Hassan M, Latif N, Yacoub M. Adipose tissue: friend or foe? Nat Rev Cardiol 2012; 9:689-702; PMID:23149834; http://dx.doi.org/10.1038/nrcardio.2012.148
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med 2009; 360:1500-8; PMID:19357405; http://dx.doi.org/10.1056/NEJMoa0808718
- Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L, Hu H, Wang L, Pavlova Z, Gilsanz V, et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One 2012; 7:e49452; PMID:23166672; http://dx.doi.org/10.1371/journal.pone.0049452
- Algire C, Medrikova D, Herzig S. White and brown adipose stem cells: from signaling to clinical implications. Biochim Biophys Acta 2013; 1831:896-904; PMID:23051608; http://dx.doi.org/10.1016/j.bbalip.2012.10.001
- Xue B, Rim JS, Hogan JC, Coulter AA, Koza RA, Kozak LP. Genetic variability affects the development of brown adipocytes in white fat but not in interscapular brown fat. J Lipid Res 2007; 48:41-51; PMID:17041251; http://dx.doi.org/10.1194/jlr.M600287-JLR200
- Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor γ (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 2010; 285:7153-64; PMID:20028987; http://dx.doi.org/10.1074/jbc.M109.053942
- Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012; 150:366-76; PMID:22796012; http://dx.doi.org/10.1016/j.cell.2012.05.016
- Enerbäck S. The origins of brown adipose tissue. N Engl J Med 2009; 360:2021-3; PMID:19420373; http://dx.doi.org/10.1056/NEJMcibr0809610
- Loncar D. Brown adipose tissue as a derivative of mesoderm grafted below the kidney capsule. A model for differentiation of isolated rat mesoderm. Int J Dev Biol 1992; 36:265-74; PMID:1525014
- Atit R, Sgaier SK, Mohamed OA, Taketo MM, Dufort D, Joyner AL, Niswander L, Conlon RA. Beta-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev Biol 2006; 296:164-76; PMID:16730693; http://dx.doi.org/10.1016/j.ydbio.2006.04.449
- Timmons JA, Wennmalm K, Larsson O, Walden TB, Lassmann T, Petrovic N, Hamilton DL, Gimeno RE, Wahlestedt C, Baar K, et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci U S A 2007; 104:4401-6; PMID:17360536; http://dx.doi.org/10.1073/pnas.0610615104
- Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scimè A, Devarakonda S, Conroe HM, Erdjument-Bromage H, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008; 454:961-7; PMID:18719582; http://dx.doi.org/10.1038/nature07182
- Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 2006; 7:885-96; PMID:17139329; http://dx.doi.org/10.1038/nrm2066
- Kanazawa A, Tsukada S, Kamiyama M, Yanagimoto T, Nakajima M, Maeda S. Wnt5b partially inhibits canonical Wnt/beta-catenin signaling pathway and promotes adipogenesis in 3T3-L1 preadipocytes. Biochem Biophys Res Commun 2005; 330:505-10; PMID:15796911; http://dx.doi.org/10.1016/j.bbrc.2005.03.007
- Choy L, Skillington J, Derynck R. Roles of autocrine TGF-beta receptor and Smad signaling in adipocyte differentiation. J Cell Biol 2000; 149:667-82; PMID:10791980; http://dx.doi.org/10.1083/jcb.149.3.667
- Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol 2011; 12:722-34; PMID:21952300; http://dx.doi.org/10.1038/nrm3198
- Zamani N, Brown CW. Emerging roles for the transforming growth factor-β superfamily in regulating adiposity and energy expenditure. Endocr Rev 2011; 32:387-403; PMID:21173384; http://dx.doi.org/10.1210/er.2010-0018
- Gupta RK, Arany Z, Seale P, Mepani RJ, Ye L, Conroe HM, Roby YA, Kulaga H, Reed RR, Spiegelman BM. Transcriptional control of preadipocyte determination by Zfp423. Nature 2010; 464:619-23; PMID:20200519; http://dx.doi.org/10.1038/nature08816
- Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 2006; 444:847-53; PMID:17167472; http://dx.doi.org/10.1038/nature05483
- Shaw JE, Ramwell PW. Release of prostaglandin from rat epididymal fat pad on nervous and hormonal stimulation. J Biol Chem 1968; 243:1498-503; PMID:4296686
- Steinberg D, Vaughan M, Nestel PJ, Bergstrom S. Effects of prostaglandin E opposing those of catecholamines on blood pressure and on triglyceride breakdown in adipose tissue. Biochem Pharmacol 1963; 12:764-6; PMID:13983712; http://dx.doi.org/10.1016/0006-2952(63)90053-4
- Chatzipanteli K, Rudolph S, Axelrod L. Coordinate control of lipolysis by prostaglandin E2 and prostacyclin in rat adipose tissue. Diabetes 1992; 41:927-35; PMID:1628767; http://dx.doi.org/10.2337/diab.41.8.927
- Kather H, Bieger W, Michel G, Aktories K, Jakobs KH. Human fat cell lipolysis is primarily regulated by inhibitory modulators acting through distinct mechanisms. J Clin Invest 1985; 76:1559-65; PMID:2997284; http://dx.doi.org/10.1172/JCI112137
- Bell-Parikh LC, Ide T, Lawson JA, McNamara P, Reilly M, FitzGerald GA. Biosynthesis of 15-deoxy-delta12,14-PGJ2 and the ligation of PPARgamma. J Clin Invest 2003; 112:945-55; PMID:12975479; http://dx.doi.org/10.1172/JCI200318012
- Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell 1995; 83:803-12; PMID:8521497; http://dx.doi.org/10.1016/0092-8674(95)90193-0
- Horrillo R, González-Périz A, Martínez-Clemente M, López-Parra M, Ferré N, Titos E, Morán-Salvador E, Deulofeu R, Arroyo V, Clària J. 5-lipoxygenase activating protein signals adipose tissue inflammation and lipid dysfunction in experimental obesity. J Immunol 2010; 184:3978-87; PMID:20207999; http://dx.doi.org/10.4049/jimmunol.0901355
- Bäck M, Sultan A, Ovchinnikova O, Hansson GK. 5-Lipoxygenase-activating protein: a potential link between innate and adaptive immunity in atherosclerosis and adipose tissue inflammation. Circ Res 2007; 100:946-9; PMID:17379835; http://dx.doi.org/10.1161/01.RES.0000264498.60702.0d
- García-Alonso V, López-Vicario C, Titos E, Morán-Salvador E, González-Périz A, Rius B, Párrizas M, Werz O, Arroyo V, Clària J. Coordinate functional regulation between microsomal prostaglandin E synthase-1 (mPGES-1) and peroxisome proliferator-activated receptor γ (PPARγ) in the conversion of white-to-brown adipocytes. J Biol Chem 2013; 288:28230-42; PMID:23943621; http://dx.doi.org/10.1074/jbc.M113.468603
- Spite M, Clària J, Serhan CN. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab 2014; 19:21-36; PMID:24239568; http://dx.doi.org/10.1016/j.cmet.2013.10.006
- Foudi N, Gomez I, Benyahia C, Longrois D, Norel X. Prostaglandin E2 receptor subtypes in human blood and vascular cells. Eur J Pharmacol 2012; 695:1-6; PMID:22964467; http://dx.doi.org/10.1016/j.ejphar.2012.08.009
- Nagai M, Tuchiya K, Kojima H. Prostaglandin E2 increases the calcium concentration in rat brown adipocytes and their consumption of oxygen. Prostaglandins 1996; 51:377-86; PMID:8873233; http://dx.doi.org/10.1016/0090-6980(96)00044-5
- Tsuboi H, Sugimoto Y, Kainoh T, Ichikawa A. Prostanoid EP4 receptor is involved in suppression of 3T3-L1 adipocyte differentiation. Biochem Biophys Res Commun 2004; 322:1066-72; PMID:15336573; http://dx.doi.org/10.1016/j.bbrc.2004.08.018
- Sinha D, Addya S, Murer E, Boden G. 15-Deoxy-delta(12,14) prostaglandin J2: a putative endogenous promoter of adipogenesis suppresses the ob gene. Metabolism 1999; 48:786-91; PMID:10381155; http://dx.doi.org/10.1016/S0026-0495(99)90180-4
- Feldon SE, O’loughlin CW, Ray DM, Landskroner-Eiger S, Seweryniak KE, Phipps RP. Activated human T lymphocytes express cyclooxygenase-2 and produce proadipogenic prostaglandins that drive human orbital fibroblast differentiation to adipocytes. Am J Pathol 2006; 169:1183-93; PMID:17003477; http://dx.doi.org/10.2353/ajpath.2006.060434
- Hossain MS, Chowdhury AA, Rahman MS, Nishimura K, Jisaka M, Nagaya T, Shono F, Yokota K. Stable expression of lipocalin-type prostaglandin D synthase in cultured preadipocytes impairs adipogenesis program independently of endogenous prostanoids. Exp Cell Res 2012; 318:408-15; PMID:22100987; http://dx.doi.org/10.1016/j.yexcr.2011.11.003
- Spite M, Hellmann J, Tang Y, Mathis SP, Kosuri M, Bhatnagar A, Jala VR, Haribabu B. Deficiency of the leukotriene B4 receptor, BLT-1, protects against systemic insulin resistance in diet-induced obesity. J Immunol 2011; 187:1942-9; PMID:21742977; http://dx.doi.org/10.4049/jimmunol.1100196
- Jakobsson PJ, Thorén S, Morgenstern R, Samuelsson B. Identification of human prostaglandin E synthase: a microsomal, glutathione-dependent, inducible enzyme, constituting a potential novel drug target. Proc Natl Acad Sci U S A 1999; 96:7220-5; PMID:10377395; http://dx.doi.org/10.1073/pnas.96.13.7220
- Chu X, Xu L, Nishimura K, Jisaka M, Nagaya T, Shono F, Yokota K. Suppression of adipogenesis program in cultured preadipocytes transfected stably with cyclooxygenase isoforms. Biochim Biophys Acta 2009; 1791:273-80; PMID:19416639; http://dx.doi.org/10.1016/j.bbalip.2009.01.022
- Kapoor M, Kojima F, Qian M, Yang L, Crofford LJ. Microsomal prostaglandin E synthase-1 deficiency is associated with elevated peroxisome proliferator-activated receptor gamma: regulation by prostaglandin E2 via the phosphatidylinositol 3-kinase and Akt pathway. J Biol Chem 2007; 282:5356-66; PMID:17186945; http://dx.doi.org/10.1074/jbc.M610153200
- Ghoshal S, Trivedi DB, Graf GA, Loftin CD. Cyclooxygenase-2 deficiency attenuates adipose tissue differentiation and inflammation in mice. J Biol Chem 2011; 286:889-98; PMID:20961858; http://dx.doi.org/10.1074/jbc.M110.139139
- Kim DH, Puri N, Sodhi K, Falck JR, Abraham NG, Shapiro J, Schwartzman ML. Cyclooxygenase-2 dependent metabolism of 20-HETE increases adiposity and adipocyte enlargement in mesenchymal stem cell-derived adipocytes. J Lipid Res 2013; 54:786-93; PMID:23293373; http://dx.doi.org/10.1194/jlr.M033894
- Madsen L, Pedersen LM, Lillefosse HH, Fjaere E, Bronstad I, Hao Q, Petersen RK, Hallenborg P, Ma T, De Matteis R, et al. UCP1 induction during recruitment of brown adipocytes in white adipose tissue is dependent on cyclooxygenase activity. PLoS One 2010; 5:e11391; PMID:20613988; http://dx.doi.org/10.1371/journal.pone.0011391
- Vegiopoulos A, Müller-Decker K, Strzoda D, Schmitt I, Chichelnitskiy E, Ostertag A, Berriel Diaz M, Rozman J, Hrabe de Angelis M, Nüsing RM, et al. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science 2010; 328:1158-61; PMID:20448152; http://dx.doi.org/10.1126/science.1186034
- Cederberg A, Grønning LM, Ahrén B, Taskén K, Carlsson P, Enerbäck S. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell 2001; 106:563-73; PMID:11551504; http://dx.doi.org/10.1016/S0092-8674(01)00474-3
- Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S, Spiegelman BM. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest 2011; 121:96-105; PMID:21123942; http://dx.doi.org/10.1172/JCI44271
- Kajimura S, Saito M. A new era in brown adipose tissue biology: molecular control of brown fat development and energy homeostasis. Annu Rev Physiol 2014; 76:225-49; PMID:24188710; http://dx.doi.org/10.1146/annurev-physiol-021113-170252
