Abstract
Recent studies have suggested that dysregulation of autophagy plays a pivotal role in tumorigenesis. Here, we determined the prognostic value of autophagy-related protein Beclin 1 in gastric cancer. A total of 153 primary gastric cancer patients were subjected to analysis of Beclin 1 expression and survival prognosis. Among them, 68 patients were assigned randomly and used as a training set to generate a cutoff score for Beclin 1 expression by receive operating characteristic (ROC) curve analysis. The ROC-generated cutoff score was subjected to analyze the association of Beclin 1 with clinical characteristics and patient outcome. In a testing set (n = 85) and overall patients (n = 153), both univariate and multivariate analysis found that low expression of Beclin 1 predicted adverse overall survival and progression-free survival for gastric cancer patients. Furthermore, in each stage of gastric cancer patients, Beclin 1 expression was a prognostic indicator in patients with stage II, III and IV. Importantly, a reverse relationship between Beclin 1 and Bcl-xL expression was demonstrated. In patients of elevated Bcl-xL expression, a subset with lower Beclin 1 expression displayed an inferior overall survival and progression-free survival than those with higher Beclin 1 expression. Thus, our data demonstrated that low expression of Beclin 1, associated with high Bcl-xL, played as an independent biomarker, contributing to a more aggressive cancer cell phenotype and poor prognosis for gastric tumor.
Introduction
Gastric cancer is one of the most common malignancies throughout the world.Citation1 Although the combination of surgery and systemic chemotherapy shows a favorable therapeutic outcome for early-staged gastric cancer patients, recurrence and distant metastasis are still the major issues for the poor survival of advanced patients.Citation2 Multiple structural and functional genetic alterations of oncogenes, tumor suppressor genes, cell-cycle regulators, cell adhesion molecules, and growth factors, are responsible for the development and progression of gastric cancer.Citation3 Supported by some prognostic biomarkers, the risk classification of gastric cancer patients could be defined more accurately.Citation4-Citation10 Therefore, it is of great clinical value to further understand the molecular mechanisms involved in gastric cancer and to identify more valuable prognostic biomarkers, not only improving poor prognosis but providing novel promising therapeutic targets.
Autophagy refers to an evolutionarily conserved, genetically controlled process that involves lysosomal degradation and recycling of proteins and cellular organelles.Citation11,Citation12 Autophagy maintains intracellular homeostasis by promoting non-apoptotic type II programmed cell death,Citation13,Citation14 or by facilitating adaptive cell survival.Citation15,Citation16 Intriguingly, autophagy defect is also reported to play a critical role in tumorigenesis. As the first identified mammalian autophagy effector, Beclin 1 is essential for the initiation of autophagy via its interaction with the class III phosphatidylinositol-3-kinase Vps34.Citation17 Indeed, Beclin 1 has been reported to be mono-allelically decreased or deleted in human ovarian, breast and prostate cancers.Citation18,Citation19 Inactivation or deficiency of Beclin 1 has also been shown to lead to a high incidence of spontaneous tumors in mice, including lymphomas, lung and liver cancers.Citation20,Citation21 Recently, Beclin 1 has been documented to possess a novel Bcl-2 homology region-3 (BH3) domain that interacts with the BH3 binding groove of both Bcl-2 and Bcl-xL.Citation22-Citation24 This binding of Bcl-2 family members inhibits Beclin 1-mediated autophagy,Citation22 which might be viewed as a convergence of apoptosis and autophagy. Simultaneous defects in autophagy and apoptosis lead to increased DNA damage and genomic instability that ultimately accelerates mammary tumorigenesis.Citation25,Citation26 Interestingly, low expression of Beclin 1 predicted a poor survival in Bcl-xL-overexpressed hepatocellular carcinoma (HCC) tissues.Citation27 These data indicate that coordination of autophagy and apoptosis plays a novel role in tumorigenesis and tumor prognosis. However, the crosstalk between autophagy and apoptosis is complex and not fully understood. Further studies are needed to examine the association of apoptosis regulator, Bcl-2 family members and autophagic protein Beclin 1 in clinical prognosis in more cancer types.
In the present study, we examined the expression of autophagic protein Beclin 1 and anti-apoptotic protein Bcl-xL in gastric cancer cell lines and cancer tissues. Our results showed that low expression of Beclin 1, as detected by immunohistochemistry and western blot analysis, positively correlated with tumor differentiation and was an independent biomarker for shortened survival time of patients with gastric cancer. Moreover, we detected a closely negative relationship between Beclin 1 and Bcl-xL expression. In the Bcl-xL high-expression subset, low expression of Beclin 1 predicted an inferior overall survival (OS) and progression-free survival (PFS) rate in the gastric cancer patients compared with those with high expression of Beclin 1.
Result
Beclin 1 and Bcl-xL expression in gastric cancer cell lines
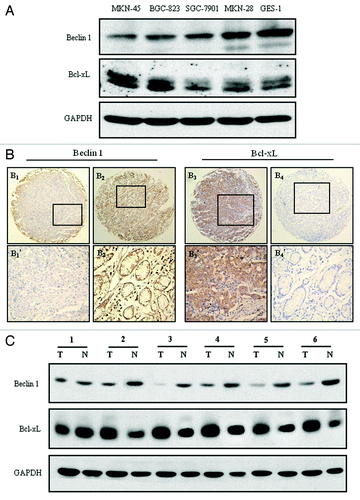
Expression of Beclin 1 and Bcl-xL in four established human gastric cancer cell lines (MKN-45, MKN-28, BGC-823 and SGC-7901) and a normal gastric mucosa epithelial cell line GES-1 were analyzed by western blot assay. In those cell lines, MKN-45 and BGC-823 cells are poorly differentiated gastric adenocarcinoma cell lines, whereas SGC-7901 and MKN-28 are moderately and well-differentiated gastric adenocarcinoma cell lines respectively.Citation28,Citation29 As shown in , Beclin 1 expression was significantly lower in the poorly differentiated cells (MKN-45 and BGC-823) than that in the moderately, well differentiated and normal mucosa epithelial cells (SGC-7901, MKN-28 and GES-1). Conversely, high expression of Bcl-xL was observed primary in poorly differentiated MKN-45 and BGC-823 cells (). These initial observations revealed that autophagy might be correlated with gastric tumor differentiation, especially under conditions in which apoptosis is compromised.
Figure 1. Beclin 1 and Bcl-xL expression in gastric cancer cell lines and tissues. (A) Western blot analysis of Beclin 1 and Bcl-xL expression in four gastric cancer cell lines (MKN-45, BGC-823, SGC-7901 and MKN-28) and a normal gastric mucosa epithelial cell line (GES-1). Equal loading of protein was determined by GAPDH. (B) Immunohistochemical staining for Beclin 1 and Bcl-xL in gastric cancer and normal adjacent mucosal tissues: (B1) Beclin 1 was moderately expressed in gastric cancer tissue, (B2) and strong stained in paired normal adjacent mucosal tissue (100 × ); (B3) Bcl-xL was overexpressed in the cytoplasm in the gastric cancer tissue, (B4) and nearly negative expressed in paired normal mucosal tissues from the same case (100 × ). (B1’ and B2’) and (B3’ and B4’) demonstrated the higher magnification (400 × ) from the area of the box in (B1 and B2) and (B3 and B4) respectively. (C) Western blot analysis of Beclin 1 and Bcl-xL expression in representative primary gastric cancer tissues (T) and normal adjacent mucosal tissues (N). Equal loading of protein was determined by GAPDH.
Expression of Beclin 1 and Bcl-xL in human gastric cancer and normal mucosal tissues
Both immunohistochemistry and western blot analysis were employed to analyze Beclin 1 and Bcl-xL expression in primary gastric cancer tissue compared with normal adjacent mucosal tissue. As shown, Beclin 1 was moderately expressed in gastric cancer tissue (’), whereas strong expressed in the normal adjacent gastric mucosal tissue (’). Conversely, in the same case, Bcl-xL was overexpressed in the gastric tumor tissue compared with the normal adjacent mucosal tissue (’). Consistent with this result, western blot analysis displayed a similar finding in gastric cancer and adjacent normal tissues ().
Figure 2. Receiver operating characteristic (ROC) curves analysis of Beclin 1 cutoff score in the training set. (A) Beclin 1 cutoff point for overall survival in the training set. (B) Beclin 1 cutoff point for progression-free survival in the training set. At each immunohistochemical score, the sensitivity and specificity for the outcome being studied were plotted, thus generating a ROC curve. Beclin 1 cutoff score for overall survival and progression-free survival was 5.65 and 5.75 respectively.
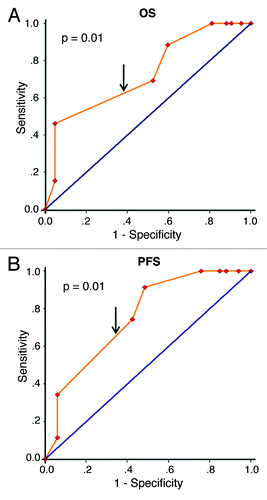
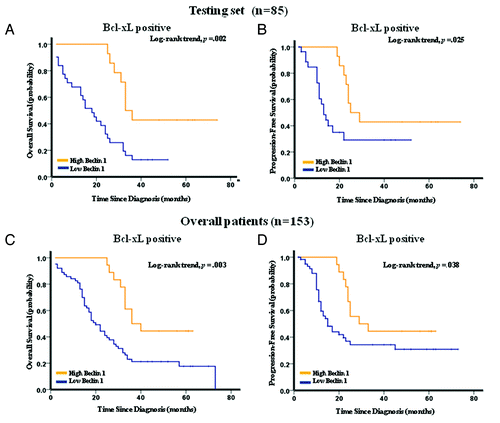
Figure 3. Kaplan-Meier survival analysis of Beclin 1 expression in the testing set and overall patients. (A) Low expression of Beclin 1 was closely correlated with poor overall survival, (B) and progression-free survival in the testing set. (C) Patients with low Beclin 1 expression also acquired an inferior overall survival, (D) and progression-free survival in overall patients. In the testing set and overall patients, the median duration of overall survival for patients with high and low expression of Beclin 1 was 78.0 vs. 15.0 mo (p < 0.001) and 82.0 vs. 22.0 mo (p < 0.001), respectively.
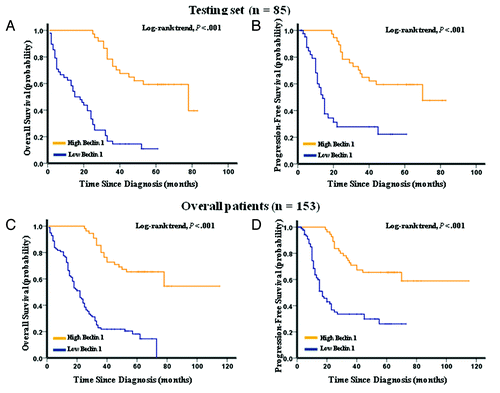
Figure 4A–F. Kaplan–Meier survival analysis of Beclin 1 expression in subsets of gastric cancer patients with stage II, III and IV. (A) Probability of overall survival and (B) progression-free survival of gastric cancer patients with stage II in the testing set: low expression, n = 6; high expression, n = 8. (C) Probability of overall survival and (D) progression-free survival of gastric cancer patients with stage III in the testing set: low expression, n = 28; high expression, n = 13. (E) Probability of overall survival and (F) progression-free survival of gastric cancer patients with stage IV in the testing set: low expression, n = 10; high expression, n = 7.
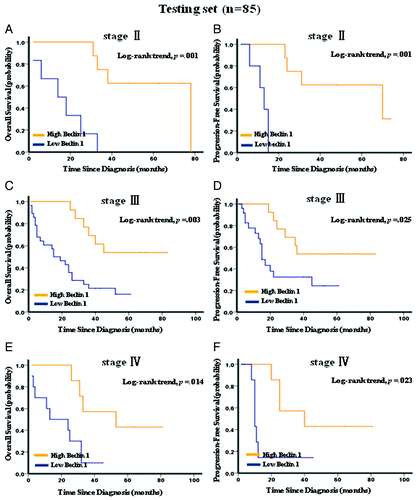
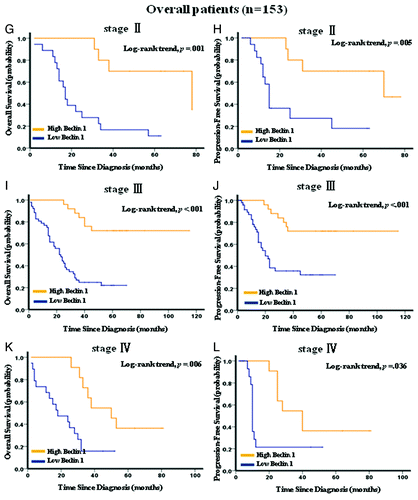
Figure 4G–L. Kaplan–Meier survival analysis of Beclin 1 expression in subsets of gastric cancer patients with stage II, III and IV. (G) Probability of overall survival and (H) progression-free survival of gastric cancer patients with stage II in the overall patients: low expression, n = 18; high expression, n = 10. (I) Probability of overall survival and (J) progression-free survival of gastric cancer patients with stage III in the overall patients: low expression, n = 52; high expression, n = 25. (K) Probability of overall survival and (L) progression-free survival of gastric cancer patients with stage IV in the overall patients: low expression, n = 19; high expression, n = 11.
To further assess survival analysis and avoid the problems of multiple cutoff point selection, ROC curve analysis was employed to determine a cutoff score for Beclin 1 expression in the training set. As shown in , the Beclin 1 cutoff score for OS and PFS in the training set (n = 68) was 5.65 (p = 0.01) and 5.75 (p < 0.001) respectively. We thus selected a Beclin 1 expression score of 5 (> 5 vs. ≤ 5) as the uniform cutoff point for survival analysis in the testing set (n = 85). Similarly, ROC analysis revealed that a score of 5 was also the cutoff point of Bcl-xL to distinguish the patients as high or low expression (Data not shown).
Beclin 1 expression and clinical features
ROC-derived Beclin 1 cutoff score of 5 in the training set segregated the testing set into high (37/85, 43.5%) and low (48/85, 56.5%) subgroups. As shown in , low expression of Beclin 1 was largely found in poorly differentiated gastric cancer tissues (72/90, 80%, in poorly differentiated tumors vs. 26/63, 41.3%, in well differentiated tumors, p < 0.001), consistent with the results described above in gastric cancer cell lines (). Further correlation analysis demonstrated that Beclin 1 was significantly positively associated with histology differentiation in both training (p = 0.013, ) and testing (p < 0.001, ) sets. In addition, low Beclin 1 expression associated with advanced clinical stage (p = 0.014, ) and tumor invasion (p = 0.037, ) in the testing set. We failed to detect any relationship between Beclin 1 with other patient characteristics, including age, gender and node stage.
Table 1. Association of Beclin 1 expression with patient’s characteristics in primary gastric cancer
Beclin 1 expression and survival analysis: Univariate survival analysis
Kaplan-Meier analysis showed that low expression of Beclin 1 strongly predicted an inferior OS and PFS in the testing set (p < 0.001 for both OS and PFS, ) and overall patients (p < 0.001 for both OS and PFS, ). Further analysis was performed with regard to Beclin 1 expression in subsets of gastric cancer patients within each clinical stage (Stage I was not included due to few patients). The results demonstrated that low expression of Beclin 1 remained a poor prognostic factor in each stage of gastric cancer patients: stage II (p = 0.001 for both OS and PFS, ), stage III (p = 0.003 for OS and p = 0.025 for PFS, ) and stage IV (p = 0.014 for OS and p = 0.023 for PFS, ). Results in the overall patients were similar to those found in the testing set ().
Association between Beclin 1, Bcl-xL expression and patient prognosis
In the total 153 gastric cancer cases studied, 81 (52.9%) patients showed high Bcl-xL expression. Interestingly, low expression of Beclin 1 was mainly found in gastric cancer patients with high expression of Bcl-xL (; ). Further correlation analysis demonstrated that a statistically significant correlation was observed between Beclin 1 and Bcl-xL expression in both training (p = 0.005, ) and testing (p = 0.014, ) sets. Moreover, the patients with high Bcl-xL expression, comprising 45 patients in the testing set, displayed a distinguished OS (p = 0.002) and PFS (p = 0.025) between subgroups with high and low Beclin 1 expression (). Similarly, among the gastric cancer patients with elevated Bcl-xL expression in the overall patients (n = 81), a subset with lower Beclin 1 also showed a worse survival compared with those with high expression of Beclin 1 (p = 0.003 for OS and p = 0.038 for PFS, ).
Figure 5. Kaplan-Meier estimation of overall survival and progression-free survival in the testing set (A and B), and overall patients (C and D), according to Beclin 1 expression in Bcl-xL positive patients. The patients with high expression of Bcl-xL, including 45 cases in the testing set and 81 cases in overall patients, were subjected to Kaplan-Meier survival analysis according to their Beclin 1 expression level. For patients with overexpression of Bcl-xL in the testing set (n = 45) and overall patients (n = 81), the median duration of overall survival for patients with high and low expression of Beclin 1 was 33 vs. 18 mo (p = 0.002), and 36 vs. 20 mo (p = 0.003) respectively.
Multivariate Cox regression analysis
To avoid the influence caused by univariate analysis, the expression of Beclin 1 as well as other parameters were examined in multivariate Cox analysis ( and ). In the testing set, Beclin 1 was indeed found to be a significant independent prognostic factor for OS (hazard ratio, 4.513; 95% CI, 2.214–9.198; p < 0.001; ) and PFS (hazard ratio, 3.168; 95% CI, 1.471–6.823; p = 0.003; ). Similar results were also observed in overall patients (hazard ratio, 4.211; 95% CI, 2.411–7.353; p < 0.001 for OS and hazard ratio, 2.623; 95% CI, 1.477–4.658; p = 0.001 for PFS; ). Moreover, histology differentiation was also identified as an independent prognostic parameter for OS and PFS in the testing set and overall patients ( and ). Of other parameters, patients’ age was evaluated as an independent prognostic factor for OS in overall patients (). However, we failed to detect that other important prognostic factors, including Bcl-xL, clinical stage and tumor invasion, were independent prognostic factors for gastric cancer, implying that larger case numbers might be needed in future studies.
Table 2. Results of multivariate Cox proportional-hazards analysis in testing set
Table 3. Results of multivariate Cox proportional-hazards analysis in overall patients
Discussion
Beclin 1, as a key regulator bridging autophagy, apoptosis and differentiation,Citation30 has been identified as a novel prognostic biomarker for ovarian,Citation31 brain,Citation32 liver,Citation27,Citation33 colon,Citation34 and nasopharyngeal carcinomas.Citation35 However, the expression dynamics of Beclin 1 and its prognostic significance in gastric cancer remain unclear. In the present study, we detected Beclin 1 expression in gastric cancer cell lines and cancer tissues. Similar to the findings in previous studies,Citation27,Citation30,Citation36 Beclin 1 was found to be lower in poorly differentiated cancer cell lines and cancer tissues, correlating closely with gastric tumor differentiation (; ). Furthermore, Beclin 1 was further validated to be an independent prognostic marker for OS and PFS in gastric cancer patients (; and ). Importantly, worse prognostic impact of low Beclin 1 expression was also demonstrated in gastric cancer patients within each clinical stage (), indicating that examination of Beclin 1 expression could be used as an additional effective tool in identifying those gastric cancer patients at increased risk of tumor progression. Taken together, our findings in this study provided evidence that decreased expression of Beclin 1 in gastric cancer patients might facilitate an increased malignant and worse prognostic phenotype of this tumor.
An interesting finding in this study is that we frequently observed low expression of Beclin 1 in gastric cancer tissues with high expression of Bcl-xL (; ). Recently, Beclin 1 has been identified as a novel BH3 protein and the relationship between Beclin 1 and Bcl-2/Bcl-xL has drawn close attention.Citation22-Citation24 Binding of Bcl-2 family members to Beclin 1 inhibited formation of the autophagy-promoting complex Beclin 1/hVps34 and decreased Beclin 1-associated class III PtdIns3K activity, leading to impairment of Beclin 1-mediated autophay.Citation22 Disrupting the binding by BH3-only proteins Bad or by BH3 mimetics compounds, has been reported to induce autophagic cell death in vitro,Citation23,Citation37-Citation39 and inhibit tumor growth in vivo,Citation38 by increasing cellular autophagy activity. Additionally, Beclin 1 was suppressed in HCC cell lines and tissues, especially when Bcl-xL was overexpressed, and low expression of Beclin 1 displayed a shortened survival time in Bcl-xL positive HCC patients.Citation27 Consistently, we found that Bcl-xL was overexpressed in various gastric cancer cell lines and cancer tissues, especially higher in those with low expression of Beclin 1 (; ). Moreover, we did observe a significantly negative correlation between Beclin 1 and Bcl-xL expression (), revealing that coordination of autophagy and apoptosis might play a novel role in gastric carcinogenesis. More importantly, we found that among patients with positive Bcl-xL expression, the subset with low Beclin 1 expression displayed a significant worse OS and PFS compared with those with elevated expression of Beclin 1. However, we failed to show that Bcl-xL was an independent prognostic biomarker for human gastric tumor ( and ). Thus, high Bcl-xL expression associates closely with Beclin 1 and contributes to a poor prognostic phenotype for gastric cancer patients.
With regard to the prognostic impact of Beclin 1 protein in various human cancer types, reports have drawn complicated conclusions. For example, decreased expression of Beclin 1 was linked to an inferior prognosis of patients with ovarian and hepatocellular carcinomas.Citation27,Citation31,Citation33 However, an elevated Beclin 1 expression was strongly correlated with poor OS, PFS and DMFS in nasopharyngeal carcinoma.Citation35 Here, we showed that low expression of Beclin 1 predicted inferior OS and PFS in gastric cancer. The distinct prognostic values of Beclin 1 might be determined by intrinsic properties of the tumor type, as well as the nature of the therapeutic regimen in various types of human cancers.Citation35 Moreover, in the present study, Beclin 1 was largely found to be lower in gastric cancer patients with Bcl-xL high expression, contributing to an adverse prognosis for these patients. Thus, distinct co-expression pattern of Beclin 1 with associated molecules may further impact disease prognosis and explain the discrepancies reported in previous studies.Citation35,Citation40
In conclusion, our study demonstrated that Beclin 1 was an independent prognostic biomarker for patient survival in gastric cancer, and Bcl-xL-associated low Beclin 1 expression might be helpful in determining the prognosis of this tumor in clinical practice. Furthermore, inhibitors of Bcl-2 family could be emerged as a novel promising cancer therapeutic strategy by inducing autophagic cell death.Citation38,Citation39 Our data suggest a novel insight into the relationship between Beclin 1, apoptosis and tumor differentiation during tumor development.
Patients and Methods
Cell culture
Human gastric cancer cell lines, including MKN-45, BGC-823, SGC-7901, MKN-28 and a human normal gastric mucosa epithelial cell line GES-1, were purchased from Shanghai Institute of Cell Biology (Shanghai, China). Cells were routinely maintained in high-glucose DMEM (Gibco, C11995) supplemented with 10% fetal bovine serum (Hyclone, SV30087.02), penicillin (100 units/mL; Sigma, P3032), and streptomycin (100 units/mL; Sigma, S9137) at 37°C in humidified 5% CO2 incubator.
Patients
Formalin-fixed, paraffin-embedded tissues from 153 patients with primary gastric cancer, who underwent initial surgical resection between March 2001 and February 2006, were collected from the archives of the Department of Pathology in the Second and Third Affiliated Hospital of Sun Yat-sen University (Guangzhou, China). All patients had follow-up records for over 5 y. The follow-up deadline was April 2011 (OS was defined as the time from diagnosis to the date of death or when censused at the latest date if patients were still alive; PFS was defined as the time from diagnosis to the date of local failure/distant metastasis or the date of death or when censused at the latest date). Of the 153 gastric cancer patients (median age, 58.0 y; range, 27–81 y; 105 male, 48 female), 100 patients were censused as death during the 5 y of follow-up time (four cases died from postoperative complications and 96 cases died from tumor progression). All patients were pathologically confirmed as adenocarcinoma of the stomach, without any metastatic diseases. Furthermore, they had no chemotherapy, radiation therapy and surgery history. Routine chemotherapy was given after resection of primary gastric tumors and no radiation treatment was administered to any of the patients. Stage and histological type were evaluated according to the WHO histological classification of gastric carcinoma. Of the overall cohort, 68 patients were randomly assigned by computer (SPSS 17.0 software) to the training set, and remaining 88 patients were randomly assigned to the testing set. This study obtained prior patients’ consent and approval from the Institute Research Ethics Committee of Sun Yat-sen University.
Tissue microarray construction
The tissue microarrays (TMAs) were constructed as a method described previously.Citation41 Briefly, individual donor tissue blocks and the corresponding histological hematoxylin and eosin–stained slides were overlaid for TMA sampling. Triplicate cylindrical tissue samples with diameter of 0.6-mm were punched from representative tumor areas and adjacent gastric mucosal tissue from individual donor tissue blocks. The tissue cylinders were then transferred to the recipient paraffin block at defined array positions by using a tissue-arraying instrument (Beecher Instruments). TMA tissue specimens for each case were composed of duplicate cylinders from gastric carcinoma tissue and one cylinder from adjacent normal mucosa tissue.
Immunohistochemical analysis and evaluation
Immunohistochemical analysis was done to study altered protein expression in 153 human gastric cancer tissues and normal adjacent mucosal tissue controls. The TMAs slides were deparaffinized in xylene, rehydrated through graded alcohol, immersed in 3% hydrogen peroxide for 10 min to block endogenous peroxidase activity. An antigen retrieval process was accomplished by pressure cooking for 3 min in Tris/EDTA (pH = 8.0). Then the slides were incubated with the primary antibody of Beclin 1 (1:200; Santa Cruz, SC-11427) and Bcl-xL (1:50; Cell Signal, 2762) for 1 h at room temperature in a moist chamber. Specimens were stained with DAB (3, 3-diaminobenzidine; Dako, K5007) after being incubated with the secondary antibody (HRP-anti-Rabbit; Thermo Scientific, 31460) for 30 min. Finally, the sections were counterstained with hematoxylin, dehydrated and mounted. Negative controls were employed by replacing the primary antibody with nonimmune serum immunoglobulins. Known immunostaining-positive slides were used as positive controls.
The brown granules in cytoplasm of Beclin 1 and Bcl-xL were considered as positive staining. For the assessment of cytoplasmic staining, the staining intensity was scored as follows:Citation35 negative (score 0), bordering (score 1), weak (score 2), moderate (score 3) and strong (score 4). Staining extent was graded into five parts according to the percentage of elevated staining cells in the field: negative (score 0), 0–25% (score 1), 26–50% (score 2), 51–75% (score 3) and 76–100% (score 4). Beclin 1 and Bcl-xL expression were evaluated by combined assessing of staining intensity and extent. The merged overall score was subjected to further survival analysis. Immunohistochemical staining was assessed and scored by two independent pathologists (Tang F and Xu J) who were blinded to the clinicopathological data. Their conclusions were in complete agreement in 85% (130/153) of the cases, suggesting that the scoring system was highly reproducible. If both of the pathologists agreed with the results they scored, the value was selected. If the results were completely different, two pathologists worked together to confirm the score.
Western blot analysis
Cells or tissues were ground and lysed with the RIPA buffer (Sigma, R0278) on ice before being subjected to western blot analysis. The protein concentration was detected by the Bradford method with BSA (Sigma, A4503) as the standard. Equal amounts of cell and tissue extract were subjected to electrophoresis in SDS-polyacrylamide gel and transferred to nitrocellulose membrane (Bio-Rad Laboratories, 162-0094) for antibody blotting. The membrane was then blocked and incubated with mouse anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody (Abmart, M20028), rabbit anti-Beclin 1 antibody (Santa Cruz, SC-11427) and rabbit anti-Bcl-xL antibody (Cell Signal, 2762).
Selection of a cutoff score for Beclin 1 expression
The ROC curve analysis was subjected to the selection of Beclin 1 cutoff score for OS and PFS in the training set, as described previously.Citation42 Briefly, the sensitivity and specificity for the outcome being studied at each Beclin 1 score was plotted to generate a ROC curve. The score localized closest to the point at both maximum sensitivity and specificity (0.0, 1.0) on the curve, was selected as the cutoff score leading to the greatest number of tumors which were correctly classified as having or not having the outcome. To facilitate ROC curve analysis, the patient outcome features were dichotomized by survival [death vs. other outcome (censored, alive, or death from other causes)] and progression (local failure or distant metastasis) (with vs. without).
Statistical analysis
For survival analysis, optimal cutoff point for Beclin 1 expression was obtained by ROC analysis in the training set (n = 68). For validation, the relationship between Beclin 1 expression, which was classified by ROC analysis-generated cutoff point, and OS, PFS were evaluated in the testing set (n = 85) and overall patients (n = 153). The chi-square test or Fisher’s exact test was employed to evaluate the relationship between Beclin 1 and clinicopathological variables. The relationships between Beclin 1 expression and OS, PFS were determined by Kaplan-Meier analysis, and differences in survival probabilities between patient subsets were assessed by the log-rank test. The multivariate Cox proportional hazards model was utilized to determine independent prognostic factors. Statistically significant difference was considered if the p value from a two-tailed test was less than 0.05. Statistical analysis was performed using SPSS v. 17.0 (SPSS, Inc.).
| Abbreviations: | ||
| ROC | = | receive operating characteristic |
| Bcl-xL | = | B-cell lymphoma-extra large |
| Bcl-2 | = | B-cell lymphoma 2 |
| BH3 | = | Bcl-2 homology region-3 |
| OS | = | overall survival |
| PFS | = | progression-free survival |
| DMFS | = | distant metastasis-free survival |
| PtdIns3K | = | phosphatidylinositol 3-kinase |
Acknowledgments
The work was supported by grants No. 30888003, 81130040 (Q.L.), 30873009 (Z.G.) and 30672409, 81071893 (C.K.S.) from the National Natural Science Foundation of China.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Dicken BJ, Bigam DL, Cass C, Mackey JR, Joy AA, Hamilton SM. Gastric adenocarcinoma: review and considerations for future directions. Ann Surg 2005; 241:27 - 39; PMID: 15621988
- Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001; 345:725 - 30; http://dx.doi.org/10.1056/NEJMoa010187; PMID: 11547741
- Zheng L, Wang L, Ajani J, Xie K. Molecular basis of gastric cancer development and progression. Gastric Cancer 2004; 7:61 - 77; http://dx.doi.org/10.1007/s10120-004-0277-4; PMID: 15224192
- Zhang XW, Sheng YP, Li Q, Qin W, Lu YW, Cheng YF, et al. BMI1 and Mel-18 oppositely regulate carcinogenesis and progression of gastric cancer. Mol Cancer 2010; 9:40; http://dx.doi.org/10.1186/1476-4598-9-40; PMID: 20170541
- de Maat MF, van de Velde CJ, Umetani N, de Heer P, Putter H, van Hoesel AQ, et al. Epigenetic silencing of cyclooxygenase-2 affects clinical outcome in gastric cancer. J Clin Oncol 2007; 25:4887 - 94; http://dx.doi.org/10.1200/JCO.2006.09.8921; PMID: 17971584
- Hayashi M, Inokuchi M, Takagi Y, Yamada H, Kojima K, Kumagai J, et al. High expression of HER3 is associated with a decreased survival in gastric cancer. Clin Cancer Res 2008; 14:7843 - 9; http://dx.doi.org/10.1158/1078-0432.CCR-08-1064; PMID: 19047113
- Chatterjee D, Sabo E, Tavares R, Resnick MB. Inverse association between Raf Kinase Inhibitory Protein and signal transducers and activators of transcription 3 expression in gastric adenocarcinoma patients: implications for clinical outcome. Clin Cancer Res 2008; 14:2994 - 3001; http://dx.doi.org/10.1158/1078-0432.CCR-07-4496; PMID: 18483365
- Zhao ZS, Wang YY, Chu YQ, Ye ZY, Tao HQ. SPARC is associated with gastric cancer progression and poor survival of patients. Clin Cancer Res 2010; 16:260 - 8; http://dx.doi.org/10.1158/1078-0432.CCR-09-1247; PMID: 20028745
- De Vita F, Giuliani F, Silvestris N, Catalano G, Ciardiello F, Orditura M. Human epidermal growth factor receptor 2 (HER2) in gastric cancer: a new therapeutic target. Cancer Treat Rev 2010; 36:Suppl 3 S11 - 5; http://dx.doi.org/10.1016/S0305-7372(10)70014-1; PMID: 21129604
- Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010; 376:687 - 97; http://dx.doi.org/10.1016/S0140-6736(10)61121-X; PMID: 20728210
- Kroemer G, Jaattela M. Lysosomes and autophagy in cell death control. Nature reviews 2005; 5:886-97.
- Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science (New York, NY 2000; 290:1717-21.
- Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature 2008; 451:1069 - 75; http://dx.doi.org/10.1038/nature06639; PMID: 18305538
- Liu Y, Schiff M, Czymmek K, Talloczy Z, Levine B, Dinesh-Kumar SP. Autophagy regulates programmed cell death during the plant innate immune response. Cell 2005; 121:567 - 77; http://dx.doi.org/10.1016/j.cell.2005.03.007; PMID: 15907470
- Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell 2005; 120:237 - 48; http://dx.doi.org/10.1016/j.cell.2004.11.046; PMID: 15680329
- Colell A, Ricci JE, Tait S, Milasta S, Maurer U, Bouchier-Hayes L, et al. GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. Cell 2007; 129:983 - 97; http://dx.doi.org/10.1016/j.cell.2007.03.045; PMID: 17540177
- Zeng X, Overmeyer JH, Maltese WA. Functional specificity of the mammalian Beclin-Vps34 PI 3-kinase complex in macroautophagy versus endocytosis and lysosomal enzyme trafficking. J Cell Sci 2006; 119:259 - 70; http://dx.doi.org/10.1242/jcs.02735; PMID: 16390869
- Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 1999; 402:672 - 6; http://dx.doi.org/10.1038/45257; PMID: 10604474
- Aita VM, Liang XH, Murty VV, Pincus DL, Yu W, Cayanis E, et al. Cloning and genomic organization of beclin 1, a candidate tumor suppressor gene on chromosome 17q21. Genomics 1999; 59:59 - 65; http://dx.doi.org/10.1006/geno.1999.5851; PMID: 10395800
- Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest 2003; 112:1809 - 20; PMID: 14638851
- Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci USA 2003; 100:15077 - 82; http://dx.doi.org/10.1073/pnas.2436255100; PMID: 14657337
- Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 2005; 122:927 - 39; http://dx.doi.org/10.1016/j.cell.2005.07.002; PMID: 16179260
- Maiuri MC, Le Toumelin G, Criollo A, Rain JC, Gautier F, Juin P, et al. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J 2007; 26:2527 - 39; http://dx.doi.org/10.1038/sj.emboj.7601689; PMID: 17446862
- Oberstein A, Jeffrey PD, Shi Y. Crystal structure of the Bcl-XL-Beclin 1 peptide complex: Beclin 1 is a novel BH3-only protein. J Biol Chem 2007; 282:13123 - 32; http://dx.doi.org/10.1074/jbc.M700492200; PMID: 17337444
- Karantza-Wadsworth V, Patel S, Kravchuk O, Chen G, Mathew R, Jin S, et al. Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Genes Dev 2007; 21:1621 - 35; http://dx.doi.org/10.1101/gad.1565707; PMID: 17606641
- Mathew R, Kongara S, Beaudoin B, Karp CM, Bray K, Degenhardt K, et al. Autophagy suppresses tumor progression by limiting chromosomal instability. Genes Dev 2007; 21:1367 - 81; http://dx.doi.org/10.1101/gad.1545107; PMID: 17510285
- Ding ZB, Shi YH, Zhou J, Qiu SJ, Xu Y, Dai Z, et al. Association of autophagy defect with a malignant phenotype and poor prognosis of hepatocellular carcinoma. Cancer Res 2008; 68:9167 - 75; http://dx.doi.org/10.1158/0008-5472.CAN-08-1573; PMID: 19010888
- Yokozaki H. Molecular characteristics of eight gastric cancer cell lines established in Japan. Pathol Int 2000; 50:767 - 77; http://dx.doi.org/10.1046/j.1440-1827.2000.01117.x; PMID: 11107048
- Zou XM, Li YL, Wang H, Cui W, Li XL, Fu SB, et al. Gastric cancer cell lines induced by trichostatin A. World J Gastroenterol 2008; 14:4810 - 5; http://dx.doi.org/10.3748/wjg.14.4810; PMID: 18720545
- Wang J. Beclin 1 bridges autophagy, apoptosis and differentiation. Autophagy 2008; 4:947 - 8; PMID: 18769161
- Shen Y, Li DD, Wang LL, Deng R, Zhu XF. Decreased expression of autophagy-related proteins in malignant epithelial ovarian cancer. Autophagy 2008; 4:1067 - 8; PMID: 18776739
- Miracco C, Cosci E, Oliveri G, Luzi P, Pacenti L, Monciatti I, et al. Protein and mRNA expression of autophagy gene Beclin 1 in human brain tumours. Int J Oncol 2007; 30:429 - 36; PMID: 17203225
- Shi YH, Ding ZB, Zhou J, Qiu SJ, Fan J. Prognostic significance of Beclin 1-dependent apoptotic activity in hepatocellular carcinoma. Autophagy 2009; 5:380 - 2; http://dx.doi.org/10.4161/auto.5.3.7658; PMID: 19145109
- Li BX, Li CY, Peng RQ, Wu XJ, Wang HY, Wan DS, et al. The expression of beclin 1 is associated with favorable prognosis in stage IIIB colon cancers. Autophagy 2009; 5:303 - 6; http://dx.doi.org/10.4161/auto.5.3.7491; PMID: 19066461
- Wan XB, Fan XJ, Chen MY, Xiang J, Huang PY, Guo L, et al. Elevated Beclin 1 expression is correlated with HIF-1alpha in predicting poor prognosis of nasopharyngeal carcinoma. Autophagy 2010; 6:395 - 404; http://dx.doi.org/10.4161/auto.6.3.11303; PMID: 20150769
- Wang J, Lian H, Zhao Y, Kauss MA, Spindel S. Vitamin D3 induces autophagy of human myeloid leukemia cells. J Biol Chem 2008; 283:25596 - 605; http://dx.doi.org/10.1074/jbc.M801716200; PMID: 18628207
- Maiuri MC, Criollo A, Tasdemir E, Vicencio JM, Tajeddine N, Hickman JA, et al. BH3-only proteins and BH3 mimetics induce autophagy by competitively disrupting the interaction between Beclin 1 and Bcl-2/Bcl-X(L). Autophagy 2007; 3:374 - 6; PMID: 17438366
- Lian J, Wu X, He F, Karnak D, Tang W, Meng Y, et al. A natural BH3 mimetic induces autophagy in apoptosis-resistant prostate cancer via modulating Bcl-2-Beclin1 interaction at endoplasmic reticulum. Cell Death Differ 2011; 18:60 - 71; http://dx.doi.org/10.1038/cdd.2010.74; PMID: 20577262
- Tian S, Lin J, Jun Zhou J, Wang X, Li Y, Ren X, et al. Beclin 1-independent autophagy induced by a Bcl-XL/Bcl-2 targeting compound, Z18. Autophagy 2010; 6:1032 - 41; http://dx.doi.org/10.4161/auto.6.8.13336; PMID: 20818185
- Chen C, Ma Q, Ma X, Liu Z, Liu X. Association of Elevated HIF-2alpha Levels with Low Beclin 1 Expression and Poor Prognosis in Patients with Chondrosarcoma. Ann Surg Oncol 2011; 18:2364 - 72; http://dx.doi.org/10.1245/s10434-011-1587-5; PMID: 21327823
- Xie D, Sham JS, Zeng WF, Lin HL, Che LH, Wu HX, et al. Heterogeneous expression and association of beta-catenin, p16 and c-myc in multistage colorectal tumorigenesis and progression detected by tissue microarray. Int J Cancer 2003; 107:896 - 902; http://dx.doi.org/10.1002/ijc.11514; PMID: 14601048
- Zlobec I, Steele R, Terracciano L, Jass JR, Lugli A. Selecting immunohistochemical cut-off scores for novel biomarkers of progression and survival in colorectal cancer. J Clin Pathol 2007; 60:1112 - 6; http://dx.doi.org/10.1136/jcp.2006.044537; PMID: 17182662