Abstract
Many fish species experience long periods of fasting often associated with seasonal reductions in water temperature and prey availability or spawning migrations. During periods of nutrient restriction, changes in metabolism occur to provide cellular energy via catabolic processes. Muscle is particularly affected by prolonged fasting as proteins of this tissue act as a major energy source. However, the molecular components involved in muscle protein degradation as well as the regulatory networks that control their function are still incompletely defined in fish. The present work aimed to characterize the response of the autophagy-lysosomal degradative pathway to nutrient and serum availability in primary culture of rainbow trout myoblasts. In this aim, 4-day-old cells were incubated in a serum and amino acid-rich medium (complete medium), a serum and amino acid-deprived medium (minimal medium) or a minimal medium plus amino acids, and both the transcription-independent short-term response and the transcription-dependent long-term response of the autophagy-lysosomal degradative pathway were analyzed. We report that serum and amino acids withdrawal is accompanied by a rapid increase of autophagosome formation but also by a slower induction of the expression of several autophagy-related genes (LC3B, gabarapl1, atg4b). We also showed that this latter response is controlled by amino acid (AA) availability and that both TOR-dependent and TOR-independent pathways are involved in this effect. Together these results suggest an important role for AA released by muscle proteolysis during the fasting period in regulating the subtle balance between using proteins as disposable furniture to provide energy, and conserving muscle through protein sparing.
Introduction
Many fish species migrate long distances to the spawning grounds. Extreme examples are the temperate eel species (2000–6000 km)Citation1,Citation2 and salmonid species (up to 3000 km).Citation3,Citation4 In the course of the migration, fish enter into a long-term starving phase and degrade almost all their lipid and about half their white muscle mass, thereby reducing their ability to burst swim.Citation5 Not all degradation products are funneled into oxidative pathways, but substantial amounts provide the building blocks for the developing oocytes, some of them mediated through hepatic vitellogenin biosynthesis. Fuel usage changes drastically, with protein increasingly important as migration progresses, and likely the sole energy source for the last half of the migration and the energetically costly spawning process itself. Carbohydrates are used intermittently, and rebuilt from amino acids for spawning.Citation5,Citation6 Overall, these data highlight the importance of a tight control of muscle protein degradation in these species not only to fuel migration but also to fuel gonadal maturation and spawning. Individual variations in this control could therefore lead to differences in reproductive success or life span. However, the molecular components involved in muscle protein degradation as well as the regulatory networks that control their function are still incompletely defined.
Protein degradation in the skeletal muscle of vertebrates is thought to be essentially mediated by the activity of two highly conserved pathways, the ubiquitin-proteasomal pathway and the autophagic/lysosomal pathway. Whereas ubiquitin-proteasome-dependent degradation has been investigated in depth and its contribution to muscle loss has been already well documented in mammals,Citation7-Citation11 the role of autophagy has only recently begun to be investigated and new evidence has demonstrated that this pathway plays a critical role in controlling muscle mass.Citation12,Citation13 In fish, the ubiquitin-proteasome system has been intensively investigated in recent years and significant progress has been made in understanding the mechanisms that control this system.Citation14-Citation20 In contrast, limited studies have focused on the autophagic/lysosomal pathway which is recognized to be upregulated during muscle wasting,Citation19,Citation21,Citation22 and the regulatory networks that control this pathway remain little understood in fish.
The autophagic/lysosomal pathway is an evolutionarily conserved process that is responsible for the degradation of long-lived proteins and for the elimination of redundant or damaged cellular structures, e.g., mitochondria. During autophagy, portions of cytoplasm and cell organelles are sequestered into vesicles, called autophagosomes, with subsequent fusion of autophagosomes with lysosomes, and digestion of the content of the vacuoles by lysosomal hydrolases.Citation23 In mammalian models, two main mechanisms have been identified for the induction of autophagosome formation in response to nutrient and growth factor starvation. The first is a rapid and transient transcription-independent induction mediated by mammalian target of rapamycin (mTOR) and AMP-activated protein kinase (AMPK) complexes, the major nutrient and energy sensors in eukaryotic cells, respectively.Citation24 The second regulatory pathway is a slower mechanism requiring gene expression and is thought to allow the persistent generation of autophagosomes during prolonged fasting.Citation13,Citation25 Recent studies in mammals have shown that the transcription factors Forkhead box-O (FoxO3a and FoxO1) are necessary and sufficient to activate autophagy by controlling the expression of some critical autophagy-related genes [Lc3b and Gabarapl1 (the mammalian Atg8-related proteins), Vps34, Ulk2 and atg12].Citation26,Citation27 FoxO proteins are also downstream targets of the insulin-like growth factor-1 (IGF1)/insulin-phosphatidylinositol 3-kinase (PtdIns3K)-protein kinase B/Akt signaling, and are thus considered to be major mediators of growth factor effect on autophagy-related gene expression.Citation26,Citation27
Recently, we have shown that 14-d fasting of rainbow trout (Oncorhynchus mykiss) strongly induces the expression of several major genes involved in autophagy (gabarapl1, LC3b, atg4b and atg12l) in white muscle.Citation22 These results are consistent with the strong activation of the lysosomal degradative system under catabolic situations, as observed by the induction of the expression and activity of cathepsins L, B, D and S during salmonid migration, maturation and starvation,Citation19,Citation21 and provide new evidence for the importance of the autophagic/lysosomal pathway for muscle atrophy in rainbow trout. However, although recent studies have shown the clear conservation of the above-mentioned signaling proteins (Akt, TOR, AMPK, FoxO) in rainbow trout,Citation15,Citation22,Citation28-Citation32 little is known about the mechanisms involved in the induction of autophagosome formation in response to nutrient- and growth factor-starvation in fish. To our knowledge, the only available data suggest that the regulatory networks that control the expression of autophagy-related genes have probably diverged throughout evolution.Citation22,Citation33 Indeed, in contrast to what is described in mammalian models, our recent results failed to demonstrate any insulin or IGF1 effect on the expression of autophagy-related genes.Citation22,Citation27 Therefore, the aim of the present study was to characterize both the transcription-independent short-term response and the transcription-dependent long-term response of the autophagy-lysosomal degradative pathway to nutrient and serum withdrawal in primary culture of rainbow trout muscle cells. Particular attention was paid to the nature of the stimuli that control the latter response, which would be of particular importance in salmonid species that, in the course of their spawning migration, enter into a long-term starving phase.
Results
Serum and amino acid withdrawal induces autophagosome formation in trout myoblasts
Rapid changes in macroautophagy have long been known to occur in response to changes in nutrient and/or hormonal (insulin/IGF1) supply.Citation34 To test whether serum and amino acid withdrawal was also accompanied by an increase of autophagosome formation in our cell culture model, we monitored by immunofluorescence the subcellular localization of the LC3 protein in cells incubated in a serum- and amino acid-rich (complete medium, CM) or -deprived medium (minimal medium, MM) for 4 h. As shown in , immunofluorescence of CM-incubated cells showed LC3 staining as a weak diffuse cytoplasmic pool. In contrast, MM-incubated cells revealed the appearance of punctate LC3 staining that represent autophagosomes,Citation35 indicating that serum and amino acid withdrawal induced autophagosome formation in our cell culture model. Furthermore, the addition of bafilomycin A1 (Baf A1), a vacuolar ATPase inhibitor that inhibits autophagosome-lysosome fusion and prevents the degradation of LC3,Citation36 had a weak effect in CM-incubated cells, but strongly induced the number of punctate LC3 structures in MM-incubated cells, further supporting the evidence of a short-term induction of autophagosome formation by serum and amino acid withdrawal in our cell culture model.
Figure 1. Effect of serum- and AA-withdrawal on autophagosome formation in trout myoblasts. Four-day-old cells were incubated in a serum- and amino acid-rich (complete medium, CM) or -deprived medium (minimal medium, MM) with or without bafilomycin A1 (Baf A1). (A) Immunolocalization of LC3B (green) was performed after 4 h incubation as described in Materials and Methods. Nuclei were stained with Hoescht (blue). Graph represents the quantification of the number of LC3 puncta per cell. Results are expressed as means ± SEM, n = 4–5 (mean of 4–5 replications) and were analyzed by one-way ANOVA followed by Student Newman–Keuls test for multiple comparison (p < 0.05). Different letters represent significantly different values. (B) After 4 h incubation, cell lysates (10 µg) were analyzed by western blot with an anti-LC3B antibody. β-actin was used as loading control. The ratio of LC3-II to β-actin is shown quantitatively as a graph. (C) Cells were incubated in a serum- and amino acid-rich (complete medium, CM) or -deprived medium (minimal medium, MM) for 0.5, 2, 4, 6, 12, 24 h. Cell lysates (10 µg) were analyzed by western blot with the indicated antibodies. A representative blot is shown. Graphs represent the ratio of LC3-II to β-actin. Results are means ± SEM, n = 3 (mean of 3 replications) and were analyzed using one way ANOVA followed by the Student Newman–Keuls test for multiple comparisons (p < 0.05). Different letters represent significantly different values.
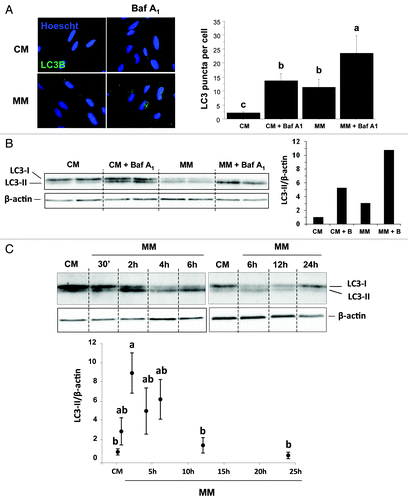
Given that autophagy is a highly dynamic and complex process that is tightly regulated at multiple steps, and in order to strengthen our conclusion, we performed in lysates from CM- and MM-incubated cells western blots for LC3B. Activation of autophagy converts LC3B from a slower migrating unconjugated form (LC3-I) to a faster migrating lipid-conjugated form (LC3-II).Citation37 As shown in , the serum and AA withdrawal was associated with an increase of the ratio LC3-II/β-actin (compare lanes CM and MM) which is a hallmark of autophagy induction. Furthermore, the LC3-II/β-actin ratio in the presence of Baf A1 is higher in MM-incubated cells (compare lanes CM+Baf A1 and MM+Baf1A1), indicating that autophagic flux is increased during serum and AA withdrawal.
Finally, in order to better characterize the autophagy flux in our cell culture model, we monitored by western blotting the LC3 lipidation in 4-d-old cells cultivated in CM and MM for 0.5, 2, 4, 6, 12 and 24 h. As shown in , the ratio of LC3-II to β-actin was significantly increased after 2 h in MM (p < 0.05) before declining to the level of the CM-incubated cells.
Overall, these results demonstrated that serum and amino acid withdrawal was accompanied by a rapid and transient increase of autophagy in our cell culture model, indicating that these serum and AA-deprived cells can serve as a relevant model to characterize the factors involved in the induction of this proteolytic system.
Serum and amino acid withdrawal induces the expression of several autophagy-related genes in trout myoblasts
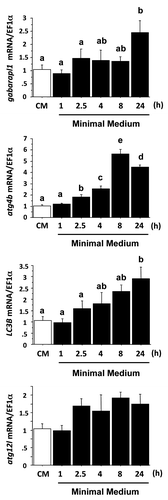
To test if a serum and AA withdrawal might also induce the expression of autophagy-related genes in our cell culture model, we analyzed by qRT-PCR the mRNA levels of several autophagy-related genes in CM- and MM-incubated cells. As shown in , among the four genes studied, atg12l was the only gene whose expression was not significantly affected by serum and amino acid withdrawal. The mRNA levels of gabarapl1 and LC3B significantly increased after 24 h of serum and AA removal. Those for atg4b reached significantly higher levels than those of CM-incubated cells as early as 2.5 h after serum and AA removal and remained higher until 24 h. Altogether, these results demonstrate that in addition to the short-term (4 h) effect on the autophagosomal formation demonstrated above, the serum and AA withdrawal allows also a long-term (24 h) induction of the expression of several autophagy-related genes in our cell culture model.
Figure 2. Effect of serum- and AA-withdrawal on autophagy-related gene expression in trout myoblasts. Four-day-old cells were incubated in a serum- and amino acid-rich (complete medium, CM) or -deprived medium (minimal medium, MM) for 1, 2.5, 4, 8 or 24 h. Gabarapl1, LC3B, atg4b and atg12l mRNA levels were estimated using real-time RT-PCR. Expression values were normalized with elongation factor 1α (EF1α)-expressed transcripts. Results are expressed as means ± SEM, (n = 3 independent experiments, each the mean of 6 replications) and were analyzed by one-way ANOVA followed by Student Newman–Keuls test for multiple comparison (p < 0.05). Means not sharing the same letter are significantly different.
AA availability controls the expression of several autophagy-related genes and the activity of several AA and growth factor sensing signaling pathways in trout myoblasts
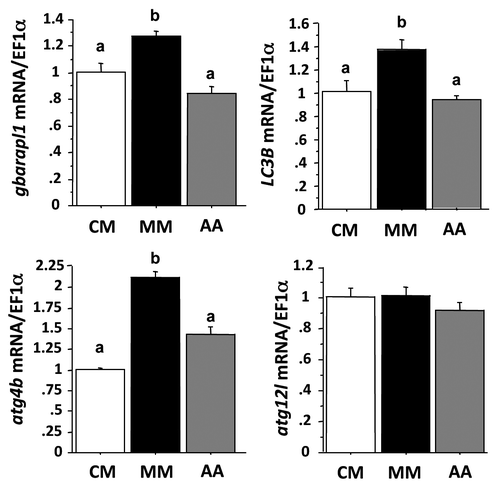
There is growing evidence that AA play an important role in controlling gene expression.Citation28,Citation29,Citation38-Citation40 In order to test if AA play a role in the above demonstrated induction of autophagy-related gene expression in MM-incubated cells, we analyzed by qRT-PCR the mRNA levels of the autophagy-related genes in CM-, MM- or MM plus AA (AA)-incubated cells for 24 h (). As presented above, serum and AA removal induced the expression of gabarapl1, LC3B and atg4b and had no effect on atg12l. The addition of AA pool into the MM medium reduced the levels of transcripts of the first three genes to that observed in CM cells, but had no effect on that of atg12l. These results demonstrated for the first time that the expression of autophagy-related genes is regulated by the availability of AA in the cells.
Figure 3. Effect of AA on autophagy-related gene expression in trout myoblasts. Four-day-old cells were incubated in a serum- and amino acid-rich (complete medium, CM) or -deprived medium (minimal medium, MM) for 1 h. The minimal medium was then replaced for 24 h with an amino acid-free (MM) or amino acids (AA)-containing medium (MEM essential and nonessential AA mixtures). LC3B, gabarapl1, atg12l and atg4b mRNA levels were estimated using real-time RT-PCR. Expression values were normalized with elongation factor 1α (EF1α)-expressed transcripts. Results are expressed as means ± SEM, (n = 3 independent experiments, each the mean of 6 replications) and were analyzed by one-way ANOVA followed by Student Newman–Keuls test for multiple comparison (p < 0.05). Different letters represent significantly different values.
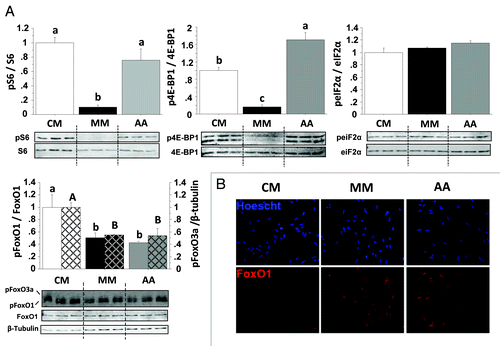
In order to clarify the mechanisms involved in the observed effect of AA on the autophagy-related gene expression we then monitored the activation of several AA and growth factor sensing signaling pathways in CM-, MM- and MM plus AA-incubated cells for 30 min () and 4 h (data not shown). The phosphorylation of the two TOR effectors S6 and the eukaryotic initiation factor 4E binding protein (4E-BP1) was strongly induced after AA addition to reach levels similar (S6) or higher (4E-BP1) that those observed in CM-incubated cells. In contrast, the phosphorylation status of eIF2α, known to play an essential role in the amino acid regulation of a large number of genes, was not affected by the treatments in the times monitored. Finally, we examined the phosphorylation status of FoxO1 and FoxO3a, which are considered to play a key role in the control of autophagy-related gene expression in mammals. As shown in , serum and AA removal decreased their phosphorylation (compare CM and MM) but the addition of AA pool into the MM medium had no effect (compare MM and AA). To further exclude FoxO action on the target genes, we then monitored by immunofluorescence the localization of FoxO1 in CM-, MM- and MM plus AA-incubated cells for 4 h. As shown in , immunofluorescence of MM- and MM plus AA-incubated cells revealed that FoxO1 colocalized with Hoescht staining, indicating that AA addition did not affect the nuclear localization of the studied protein. In contrast, CM-incubated cells showed the loss of nuclear staining for FoxO1, indicating that this treatment prevents nuclear translocation of FoxO1.
Figure 4. Effect of AA on the phosphorylation of S6, 4E-BP1, eIF2α, FoxO1 and FoxO3a proteins in trout myoblasts. (A) Four-day cultivated cells were incubated in a serum- and AA-deprived medium for 1 h. Afterwards, the culture medium was replaced with a serum- and AA-rich medium (complete medium group, CM), a serum- and AA-free medium (minimal medium group, MM) or a medium containing amino acids (Amino acids group AA) for 30 min before harvest. Cell lysates (10µg) were analyzed by western blot with the indicated antibodies. A representative blot is shown. Graphs represent the ratio between the phosphorylated protein and the total amount of the targeted protein. For FoxO proteins, filled and dashed columns represent FoxO1 and FoxO3a, respectively. Results are means ± SEM, n = 3 (mean of 3 replications) and were analyzed using one way ANOVA followed by the Student Newman–Keuls test for multiple comparisons (p < 0.05). Different letters represent significantly different values. (B) Immunolocalization of FoxO1 (red) was performed as described in Materials and Methods. Nuclei were stained with Hoescht (blue).
Overall, these results demonstrated that AA availability controls the expression of several autophagy-related genes as well as the activation of the protein TOR in our cell culture model. These results ask the question of the involvement of this protein in the above demonstrated effect of AA on autophagy-related gene expression.
AA control the expression of several autophagy-related genes via TOR-dependent and -independent mechanisms
Recent findings demonstrate the involvement of the complex TORC1 in the regulation of the expression of several metabolic- and growth-related genes.Citation41 In order to determine whether TORC1 mediates the above presented effect of AA addition on the expression of autophagy-related genes, MM-incubated cells were stimulated with AA in the presence or absence of the TOR inhibitor rapamycin for 30 min, 4 h and 24 h. We first checked the specificity of rapamycin treatment in our cell culture model by monitoring by western blot the activation of several TOR-dependent and -independent signaling pathways in cells stimulated for 30 min () and 4 h (data not shown). Whatever the time monitored, the AA-mediated induction of phosphorylation of the two TOR effectors S6 and 4EBP1 was strongly abolished in the presence of rapamycin. In contrast, the phosphorylation of eIF2α and FoxO proteins was not affected by rapamycin treatment. Overall, these results demonstrated that rapamycin treatment specifically inhibits the activity of TOR in our cell culture model.
Figure 5. Effect of AA with or without rapamycin on the phosphorylation of S6, 4E-BP1, eIF2α, FoxO1 and FoxO3a proteins in trout myoblasts. Four-day cultivated cells were incubated in a serum- and AA-deprived medium for 30 min and then preincubated for 30 min with or without 100 nM rapamycin. Afterwards, the culture mediums were replaced for 30 min with a serum- and AA-rich medium (complete medium group, CM), a serum- and AA-free medium (minimal medium group, MM; rapamycin group, R) or a medium containing amino acids (amino acids group AA and amino acids plus rapamycin group AAR). Cell lysates (10 µg) were analyzed by western blot with the indicated antibodies. A representative blot is shown. Graphs represent the ratio between the phosphorylated protein and the total amount of the targeted protein. For FoxO proteins, filled and dashed columns represent FoxO1 and FoxO3a, respectively. Results are means ± SEM, n = 3 (mean of 3 replications) and were analyzed using one way ANOVA followed by the Student Newman–Keuls test for multiple comparisons (p < 0.05). Different letters represent significantly different values.
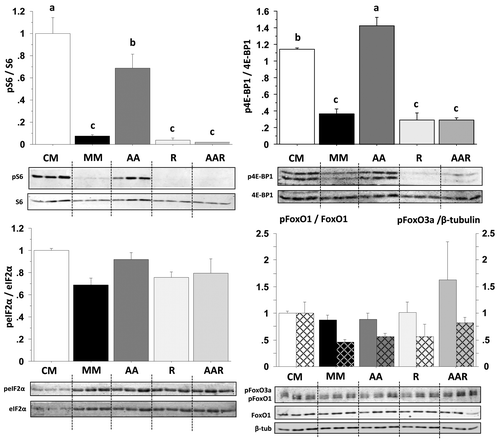
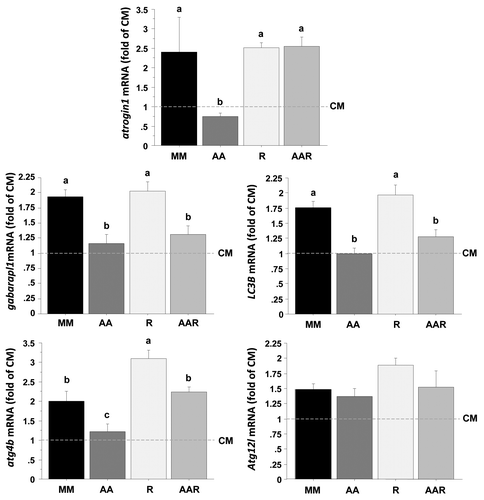
We then analyzed by qRT-PCR the mRNA levels of autophagy-related genes in CM- and MM-incubated cells stimulated with or without AA in the presence or absence of rapamycin for 24 h (). The expression of atrogin1 is downregulated by AA via a TOR-dependent mechanismCitation39,Citation40 and was therefore monitored as a control. As expected, atrogin1 was strongly downregulated (> 2-fold) after 24 h of stimulation with AA, but not with AA plus rapamycin. In contrast, the effect of AA on the expression of gabarapl1 and LC3B genes was not abolished in the presence of rapamycin, suggesting that AA act on these genes via a TOR-independent way. Finally, the AA-mediated downregulation of the expression of the atg4b gene was significantly affected by rapamycin treatment, supporting an involvement of the TORC1 complex in the observed effect of AA on the expression of this gene. Taken together, these results demonstrate for the first time that AA regulate the expression of several autophagy-related genes via mechanisms involving both TOR-dependent and TOR-independent pathways.
Figure 6. Effect of AA with or without rapamycin on the expression of several autophagy-related genes in trout myoblasts. Four-day cultivated cells were incubated in a serum- and AA-deprived medium for 30 min and then preincubated for 30 min with or without 100 nM rapamycin. Afterwards, the culture mediums were replaced for 24 h with a serum- and AA-rich medium (complete medium group, CM), a serum- and AA-free medium (minimal medium group, MM; rapamycin group, R) or a medium containing amino acids (amino acids group AA and amino acids plus rapamycin group AAR). Atrogin1, LC3B, gabarapl1, atg12l and atg4b mRNA levels were estimated using real-time RT-PCR. For each treatment, six replicates were performed. Expression values were normalized with Rps29 expressed transcripts. Results are expressed as fold of the CM group and presented as means ± SEM, n = 6 (mean of 6 replications). They were analyzed by one-way ANOVA followed by Student Newman–Keuls test for multiple comparison (p < 0.05). Different letters represent significantly different values.
Discussion
In mammals, new evidence has demonstrated the important role of the autophagic/lysosomal pathway in regulating muscle mass and identified the transcription factors FoxO as a key factor of the control of this proteolytic system in response to nutrient and growth factor starvation by inducing several autophagy-related genes.Citation26,Citation27 In rainbow trout, our previous findings showed that fasting fish for 14 d or serum depletion of trout myoblasts strongly induces the expression of several autophagy-related genes.Citation22 However, we failed to link the phosphorylation status of FoxOs with the expression of autophagy-related genes, suggesting a moderate role for this transcription factor on the autophagic/lysosomal pathway in this species. The aim of the present study was therefore to clarify the signal transduction pathway involved in the regulation of the expression of autophagy-related genes in rainbow trout and monitor the potential role of amino acids in this control by exploring the regulation of the expression of the gabarapl1, LC3B, atg12l and atg4b genes in a culture of rainbow trout myoblasts.
Short- and long-term induction of the autophagy-lysosomal degradative pathway by serum and AA withdrawal in trout myoblasts
In mammals, rapid changes in the autophagy-lysosomal degradative pathway have long been known to occur in response to changes in nutrient and/or hormonal (insulin/IGF1) supply.Citation34,Citation42 In the present study we demonstrated that serum- and amino acid-withdrawal was also accompanied by a short-term increase of autophagosome formation in our cell culture model, as evidenced by the appearance of punctate LC3 structures as well as the increase of LC3 processing (a reliable marker of autophagosome formationCitation43) in MM-incubated cells. This rapid induction of autophagosome formation in starved cells could be explained by its critical role in counteracting nutrient deprivation by delivering damaged or long-lived proteins, macromolecules and organelles within the lysosomal degradative machinery to generate nutrients and energy levels compatible with cell survival.Citation44,Citation45
In addition to this rapid transcription-independent induction of autophagosome formation in response to nutrient- and growth factor-starvation, a slower mechanism requiring gene expression has been described.Citation26,Citation27 Here, we demonstrated that several autophagy-related genes were induced in MM-incubated cells well after (24 h) the short-term (4 h) increase of autophagosome formation. These results are consistent with previous findings in mammals showing that during fasting, skeletal muscle shows a persistent generation of autophagosomes that continues for days.Citation25 Such prolonged autophagic induction requires transcriptional control in order to replenish LC3 and Gabarap, critical proteins that are destroyed following autophagosome fusion with the lysosome. Altogether, these results demonstrated an evolutionary conservation of the mechanisms involved in the induction of the autophagy-lysosomal system between lower and higher vertebrates, and indicated that these serum- and AA-deprived trout myoblasts may serve as a relevant model to characterize the factors involved in the transcriptional regulation of autophagy genes.
Role of AA in the control of the expression of autophagy-related genes
Amino acids are not only substrates for various metabolic pathways, but can also serve as signaling molecules controlling signal transduction pathways.Citation46,Citation47 In addition, there is growing evidence indicating that amino acids play an important role in controlling gene expression through different pathways including at least GCN2 and TORC1.Citation28,Citation29,Citation38-Citation40,Citation48,Citation49 In a previous study, we reported that a pool of AA was able to regulate the expression of several carbohydrate- and lipid metabolism-related genes in rainbow trout hepatocytes.Citation29 Here, we demonstrated that AA availability controls also the mRNA levels of several autophagy-related genes in trout myocytes. These results indicate that in addition to the short-term inhibitory effect previously described,Citation44,Citation50 AA exert also a long-term effect on the autophagy proteolytic system. Consequently, they suggest that the amount of factors controlling the formation of the autophagosome is tightly tuned to and by environmental conditions. Whether this long-term effect of AA on autophagy-related genes is specific to the studied species, which displays very unusual features of its protein metabolism compared with mammals (i.e., a high dietary amino acid requirement and a continuous growth of the skeletal muscle throughout their lifeCitation51,Citation52) is worth investigating.
Mechanisms underlying the action of amino acids on the expression of autophagy-related genes
To our knowledge, no findings have been published to date on the effects of AA on the regulation of the expression of autophagy-related genes and the mechanisms involved remained to be determined. In this aim, we monitored the effect of AA availability on several key proteins of the main AA and growth factor-sensing signaling pathways in our cell culture model. Recent studies in mammals have shown that FoxO1 and FoxO3a are necessary and sufficient to activate autophagy by controlling the expression of some critical autophagy-related genes.Citation26,Citation27 We therefore monitored their phosphorylation status in our cell cultures and did not observe any effect of AA supplementation, excluding a possible involvement of these proteins in the observed inhibition of expression of studied genes by AA. Similarly, our results show no effect of AA availability on the phosphorylation status of eIF2α at the monitored time, also rejecting a possible contribution of the GCN2 pathway in the AA-mediated downregulation of autophagy-related genes. In contrast, we demonstrate here that AA addition leads to induction of the phosphorylation of the two TOR effectors S6 and 4E-BP1, suggesting that the TOR protein is activated in these conditions and making possible its involvement in the observed effect of AA on the expression of autophagy-related genes. Indeed, numerous previous studies revealed the importance of the TOR protein in mediating the effect of AA on the expression of several metabolic and growth-related genes.Citation28,Citation29,Citation39,Citation40,Citation49 Here, we demonstrated that the treatment of trout myocytes with the TOR inhibitor rapamycin did not prevent the inhibition of the expression of gabarapl1 and LC3B genes by AA but significantly affected that of atg4b. This indicates that AA act on the expression of autophagy-related genes via both TOR-dependent and -independent ways. In this regard, new findings have provided direct evidence on the role of several other factors in mediating the induction of the expression of autophagy-related genes in muscle wasting.Citation53 Collectively, our results and those previously published highlight the complexity of mechanisms involved in the control of the expression of autophagy-related genes. Further studies are warranted to follow these different mechanisms to see how they are affected by AA availability in trout myocytes.
Conclusions and physiological significance
In conclusion, in the present study we demonstrated the existence of both short- and long-term control of the autophagy-lysosomal degradative system in rainbow trout myoblasts. From an evolutionary adaptative point of view, this is of particular importance in salmonids that, in the course of their spawning migration, enter into a long starving phase and degrade about half of their white muscle mass. Such a prolonged degradation of muscle proteins requires transcriptional control in order to replenish critical proteins that are destroyed during autophagosome fusion with the lysosome. We also demonstrated that AA availability controls the mRNA levels of several autophagy-related genes in this species. To our knowledge, such effects of AA on the regulation of the expression of autophagy-related genes have not been demonstrated so far and whether this effect is specific to the studied species is worth investigating. However, such a regulation system could originate from an evolutionary adaptation of migrating salmonids that have to deal between energy supply (mainly coming from muscle protein-bound AA) and muscle protein sparing to achieve their spawning migration. Amino acids released by muscle proteolysis would thus regulate themselves this subtle balance that allows salmonids to finally arrive at spawning grounds. Studies along these lines either with migrating salmonids or those under experimentally induced starvation are necessary to verify our hypotheses.
Materials and Methods
Animals
Juvenile immature rainbow trout (~5 g) were maintained in our own experimental facilities (INRA, Donzacq, France) at 18°C under natural photoperiods (12 h/12 h). All experiments were performed in accordance with legislation governing the ethical treatment of animals (Decret N° 2001-464, May 29, 2001), and investigators were certified by the French government to carry out animal experiments (N° agrément 35-47). All animal work was approved by the Ministere de l'Enseignement Superieur et de le Recherche (Autorisation N° A352386).
Myosatellite cell isolation and culture
Myoblasts were performed as follows: for each culture, 30 to 60 animals, each weighing approximately 5 g, were killed by a blow to the head and then immersed for 30 sec in 70% ethanol to sterilize external surfaces. Cells were isolated, pooled and cultured following previously described protocols.Citation22,Citation54 Briefly, after removal of the skin, dorsal white muscle was isolated under sterile conditions and collected in Dulbecco's modified Eagle's medium (DMEM) containing 9 mM NaHCO3, 20 mM HEPES, 15% horse serum, and antibiotic-antimycotic cocktail [100 U/ml penicillin, 100 μg/ml streptomycin, 0.25 g/ml fungizone (Sigma, A5955)] at pH 7.4. After mechanical dissociation of the muscle in small pieces, the tissue was enzymatically digested with a 0.2% collagenase (Sigma, C-9891) solution in DMEM for 1 h at 18°C and gentle shaking. The suspension was centrifuged (300 g for 5 min at 15°C) and the resulting pellet was subjected to two rounds of enzymatic digestion with a 0.1% trypsin solution in DMEM for 20 min at 18°C with gentle agitation. After each round of trypsinization the suspension was centrifuged and the supernatant was diluted in 2 volumes of cold DMEM supplemented with 15% horse serum (Sigma, H1270) and the same antibiotic-antimycotic cocktail mentioned above. After two washes with DMEM, the cellular suspension was filtered through 100- and 40-μm nylon filters. All experiments were conducted three times with cells seeded at a density of 1.5 to 2 × 106 per well, in six-well plastic plates (Nunc, 140675). Plates and coverslips were previously treated with poly-l-lysine (Sigma, P6282) and laminin (Sigma, L2020) to facilitate satellite cell adhesion. Cells were incubated at 18°C, the optimal temperature for culture of trout origin, with a complete medium (CM) containing Earle's Balanced Salt (EBSS) culture medium (Sigma, E7510) supplemented with 10% fetal bovine serum (Sigma, F7524), MEM vitamins solution (Invitrogen, 11120-037), MEM essential amino acid mixture (Invitrogen, 11130-036) and MEM nonessential amino acid mixture (Invitrogen, 11140-035) and antibiotic-antimycotic cocktail under an air atmosphere. The medium was renewed every 2 d and observations of morphology were regularly made to control the state of the cells.
Treatment conditions
In the first experiment, 4-d old cells (myoblasts, as verified by visual microscopy) were incubated in a serum and amino acid-rich (complete medium) or -deprived medium (minimal medium) with or without Baf A1, for different times specified in the figure legends. In the following experiments, after 4 d of culture, the cells were subjected or not to serum and amino acid deprivation in a minimal medium (MM) for 1 h. Then, according to each experimental design, cells were incubated in either amino-acids free (MM) or amino acids (AA) containing medium (MEM essential and nonessential AA mixtures) for different times specified in figure legends. In experiments involving rapamycin (specific TOR-inhibitor), the inhibitor (Cell Signaling Technologies, 9904) was added 30 min prior stimulation with AA. At the end of the stimulation periods, the media were removed, the wells were washed with ice-cold PBS and the cells were used for immunofluorescence, gene expression or western blot analysis. Each experiment was performed three times.
Immunofluorescence analysis
Cells on glass coverslips were briefly washed two times by PBS and fixed 10 min with methanol. For permeabilization cells were incubated 3 min in 0.1% Triton X-100/PBS. After three washes, cells were saturated for 1 h with 3% BSA, 0,1% Tween 20 in PBS (PBST). Cells were incubated 3 h with the primary antibody anti-LC3B (Cell Signaling Technologies, 2775) diluted in blocking buffer. The secondary antibody anti-Rabbit Alexa488 (Invitrogen, A-21441) was diluted in PBST and applied for 1 h. Cells were mounted with Mowiol 4-88 (Calbiochem, 475904) containing Hoescht (0.5 μg/ml). Cells were photographed using a Canon digital camera coupled to a Canon 90i microscope.
Gene expression analysis
Total RNA samples were extracted from muscles using Trizol reagent (Invitrogen, 15596018) according to the manufacturer's recommendations. One microgram of the resulting total RNA was reverse transcribed into cDNA using the SuperScript III RNaseH-Reverse Transcriptase kit (Invitrogen, 18080085) and oligo dT (Promega, C1101) or random primers (Promega, C1181) according to the manufacturer's instructions.
Target gene expression levels were determined by real-time quantitative RT-PCR (qRT-PCR) with 300 nM of each primer. Primers were designed to overlap an intron if possible (Primer3 software) using known sequences in trout nucleotide databases (www.sigenae.org) as previously described.Citation22 Real-time RT-PCR was performed on an iCycler iQ TM real time PCR detection system using iQ TM SYBR® Green Supermix (BIO-RAD, 172-5006). Relative quantification of the target gene transcripts with a chosen reference gene transcript (EF1α and Rps29) was made following the Pfaffl method with the Relative Expression Software tool (REST©).Citation55,Citation56 In experiments involving rapamycin, Rps29 was used as reference transcripts because EF1α RNA was not stable during the experimental conditions. PCR was performed using 10 μl of the diluted cDNA mixed with five pmoles of each primer in a final volume of 25 μl. The PCR protocol was initiated at 95°C for 3 min for initial denaturation of the cDNA and hot-start iTaq TM DNA polymerase activation and continued with a two-step amplification program (20 sec at 95°C followed by 30 sec at specific primer hybridization temperature) repeated 40 times. Melting curves were systematically monitored (temperature gradient at 0.5°C/10 sec from 55 to 94°C) at the end of the last amplification cycle to confirm the specificity of the amplification reaction. The different PCR products were initially checked by sequencing to confirm the nature of the amplicon. Each PCR run included replicate samples (duplicate of reverse transcription and duplicate of PCR amplification) and negative controls (reverse transcriptase-free samples, RNA-free samples).
Protein extraction and western blotting
Protein homogenates from cells were prepared as previously described.Citation31 Protein concentrations were determined with the Bradford reagent method.Citation57 Cell lysates (10 μg of protein) were subjected to SDS-PAGE and western blotting using the appropriate antibody: Anti-phospho FoxO1 (Thr24)/FoxO3a (Thr32) (Cell Signaling Technologies, 9464), anti-FoxO1 (Epitomics, 1874-1), anti-anti-phosho S6 (Ser235/Ser236) (Cell Signaling Technologies, 4856), anti-carboxyl terminal S6 (Cell Signaling Technologies, 2217), anti-phospho 4E-BP1 (Thr37/Thr46) (Cell Signaling Technologies, 9459), anti-4E-BP1 (Cell Signaling Technologies, 9452), anti-phospho eIF2α (Ser51) (Cell Signaling Technologies, 3398), anti-carboxyl terminal eIF2α (Cell Signaling Technologies, 9722), anti-LC3B (Cell Signaling Technologies, 2775), anti-β-actin (Santa-Cruz Biotechnology, sc-47778), anti-β-tubulin (Cell Signaling Technologies, 2146). Anti-phospho FoxO1 (Thr24)/FoxO3a (Thr32), anti-FoxO1, anti-phosho S6 (Ser235/Ser236), anti-carboxyl terminal S6, anti-phospho 4E-BP1 (Thr37/Thr46), anti-4E-BP1, anti-β-tubulin and anti-β-actin have been previously validated in trout.Citation15,Citation20,Citation22,Citation28,Citation29,Citation31-Citation33 For anti-phospho eiF2α, anti-carboxyl terminal eIF2α and anti-LC3B the amino acid sequences of the corresponding proteins were monitored in the SIGENAE databaseCitation58 to check for well-conservation of the antigen sequences with the corresponding sequences from mammals, ensuring a good specificity of the used mammalian antibodies in our samples. Preliminary experiments were also performed with the murine C2C12 cell line as control. After washing, membranes were incubated with an IRDye infrared secondary antibody (LI-COR, Inc., 956-32221). Bands were visualized by Infrared Fluorescence using the Odyssey® Imaging System and quantified by Odyssey infrared imaging system software (Application software, version 1.2).
Statistical analysis
The results of gene expression and protein phosphorylation analyses were expressed as means ± SEM (n = 3 independent observations, each the mean of 6 and 3 replications for gene expression and protein phosphorylation, respectively) and analyzed by one-way ANOVA followed by Student Newman–Keuls test. For all statistical analyses, the level of significance was set at p < 0.05.
| Abbreviations: | ||
| AA | = | amino acids |
| AAR | = | serum-deprived medium containing amino acids plus rapamycin |
| AMPK | = | AMP-activated protein kinase |
| Atg4b | = | autophagy-related 4b |
| Atg12l | = | autophagy-related 12-like |
| Baf A1 | = | bafilomycin A1 |
| CM | = | complete medium |
| EF1α | = | elongation factor-1α |
| eIF2α | = | eukaryotic initiation factor-2alpha |
| 4E-BP1 | = | eukaryotic initiation factor 4E binding protein |
| FoxO | = | forkhead box-O transcription factor |
| Gabarapl1 | = | gamma-aminobutyric acid type A [GABA(A)] receptor-associated protein-like 1 |
| GCN2 | = | general control nonderepressible 2 |
| IGF1 | = | insulin-like growth factor-1 |
| LC3B | = | microtubule-associated protein 1 light chain 3B |
| MM | = | minimal medium |
| PtdIns3K | = | phosphatidylinositol 3-kinase |
| R | = | serum and amino acids deprived medium containing rapamycin |
| S6 | = | ribosomal protein S6 |
| TOR | = | target of rapamycin |
| TORC1 | = | TOR complex 1 |
Acknowledgments
We thank F. Terrier, Y. Hontang and F. Sandres for fish rearing in the INRA experimental farm (Donzacq, France). This study was supported by the French National Research Agency (ANR-08-JCJC-0025, Low utilization of dietary carbohydrates in carnivorous rainbow trout: role of amino acids, glucose and insulin interactions).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Aoyama J. Life History and Evolution of Migration in Catadromous Eels (Genus Anguilla). AquaBioScience Monographs 2009; 2:1 - 42
- Tsukamoto K. Oceanic migration and spawning of anguillid eels. J Fish Biol 2009; 74:1833 - 52; http://dx.doi.org/10.1111/j.1095-8649.2009.02242.x; PMID: 20735675
- Miller KM, Schulze AD, Ginther N, Li S, Patterson DA, Farrell AP, et al. Salmon spawning migration: Metabolic shifts and environmental triggers. Comp Biochem Physiol Part D Genomics Proteomics 2009; 4:75 - 89; http://dx.doi.org/10.1016/j.cbd.2008.11.002; PMID: 20403740
- Ueda H. Physiological mechanisms of salmon imprinting and homing migration. 16th Int Congr of Comp Endocrinol. Hong Kong, China, 2009.
- Mommsen TP, French CJ, Hochachka PW. Sites and patterns of protein and amino-acid utilization during the spawning migration of salmon. Can J Zool-Rev Can Zool 1980; 58:1785 - 99; http://dx.doi.org/10.1139/z80-246
- French CJ, Hochachka PW, Mommsen TP. Metabolic organization of liver during spawning migration of sockeye salmon. Am J Physiol 1983; 245:R827 - 30; PMID: 6660327
- Attaix D, Taillandier D. The critical role of the ubiquitin-proteasome pathway in muscle wasting in comparison to lysosomal and Ca2+-dependent systems. In: Bittar EE, Rivett AJ, eds. Advances in Molecular and Cell Biology. Stamford (USA), 1998:235-66.
- Jagoe RT, Goldberg AL. What do we really know about the ubiquitin-proteasome pathway in muscle atrophy?. Curr Opin Clin Nutr Metab Care 2001; 4:183 - 90; http://dx.doi.org/10.1097/00075197-200105000-00003; PMID: 11517350
- Kumamoto T, Fujimoto S, Ito T, Horinouchi H, Ueyama H, Tsuda T. Proteasome expression in the skeletal muscles of patients with muscular dystrophy. Acta Neuropathol 2000; 100:595 - 602; http://dx.doi.org/10.1007/s004010000229; PMID: 11078210
- Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, et al. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J 2004; 18:39 - 51; http://dx.doi.org/10.1096/fj.03-0610com; PMID: 14718385
- Lecker SH, Solomon V, Mitch WE, Goldberg AL. Muscle protein breakdown and the critical role of the ubiquitin-proteasome pathway in normal and disease states. J Nutr 1999; 129:227S - 37S; PMID: 9915905
- Masiero E, Agatea L, Mammucari C, Blaauw B, Loro E, Komatsu M, et al. Autophagy is required to maintain muscle mass. Cell Metab 2009; 10:507 - 15; http://dx.doi.org/10.1016/j.cmet.2009.10.008; PMID: 19945408
- Sandri M. Autophagy in skeletal muscle. FEBS Lett 2010; 584:1411 - 6; http://dx.doi.org/10.1016/j.febslet.2010.01.056; PMID: 20132819
- Cleveland BM, Evenhuis JP. Molecular characterization of atrogin-1/F-box protein-32 (FBXO32) and F-box protein-25 (FBXO25) in rainbow trout (Oncorhynchus mykiss): Expression across tissues in response to feed deprivation. Comp Biochem Physiol B Biochem Mol Biol 2010; 157:248 - 57; http://dx.doi.org/10.1016/j.cbpb.2010.06.010; PMID: 20601059
- Cleveland BM, Weber GM. Effects of insulin-like growth factor-I, insulin, and leucine on protein turnover and ubiquitin ligase expression in rainbow trout primary myocytes. Am J Physiol Regul Integr Comp Physiol 2010; 298:R341 - 50; http://dx.doi.org/10.1152/ajpregu.00516.2009; PMID: 20007517
- Cleveland BM, Weber GM, Blemings KP, Silverstein JT. Insulin-like growth factor-I and genetic effects on indexes of protein degradation in response to feed deprivation in rainbow trout (Oncorhynchus mykiss). Am J Physiol Regul Integr Comp Physiol 2009; 297:R1332 - 42; http://dx.doi.org/10.1152/ajpregu.00272.2009; PMID: 19726716
- Dobly A, Martin SA, Blaney SC, Houlihan DF. Protein growth rate in rainbow trout (Oncorhynchus mykiss) is negatively correlated to liver 20S proteasome activity. Comp Biochem Physiol A Mol Integr Physiol 2004; 137:75 - 85; http://dx.doi.org/10.1016/j.cbpb.2003.09.002; PMID: 14720593
- Martin SA, Blaney S, Bowman AS, Houlihan DF. Ubiquitin-proteasome-dependent proteolysis in rainbow trout (Oncorhynchus mykiss): effect of food deprivation. Pflugers Arch 2002; 445:257 - 66; http://dx.doi.org/10.1007/s00424-002-0916-8; PMID: 12457246
- Salem M, Kenney PB, Rexroad CE 3rd, Yao J. Microarray gene expression analysis in atrophying rainbow trout muscle: a unique nonmammalian muscle degradation model. Physiol Genomics 2006; 28:33 - 45; http://dx.doi.org/10.1152/physiolgenomics.00114.2006; PMID: 16882886
- Seiliez I, Panserat S, Skiba-Cassy S, Fricot A, Vachot C, Kaushik S, et al. Feeding status regulates the polyubiquitination step of the ubiquitin-proteasome-dependent proteolysis in rainbow trout (Oncorhynchus mykiss) muscle. J Nutr 2008; 138:487 - 91; PMID: 18287354
- Mommsen TP. Salmon spawning migration and muscle protein metabolism: the August Krogh principle at work. Comp Biochem Physiol B Biochem Mol Biol 2004; 139:383 - 400; http://dx.doi.org/10.1016/j.cbpc.2004.09.018; PMID: 15544963
- Seiliez I, Gutierrez J, Salmeron C, Skiba-Cassy S, Chauvin C, Dias K, et al. An in vivo and in vitro assessment of autophagy-related gene expression in muscle of rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol B Biochem Mol Biol 2010; 157:258 - 66; http://dx.doi.org/10.1016/j.cbpb.2010.06.011; PMID: 20601058
- Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell 2005; 120:237 - 48; http://dx.doi.org/10.1016/j.cell.2004.11.046; PMID: 15680329
- Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 2011; 13:132 - 41; http://dx.doi.org/10.1038/ncb2152; PMID: 21258367
- Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell 2004; 15:1101 - 11; http://dx.doi.org/10.1091/mbc.E03-09-0704; PMID: 14699058
- Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, et al. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab 2007; 6:458 - 71; http://dx.doi.org/10.1016/j.cmet.2007.11.001; PMID: 18054315
- Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, et al. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab 2007; 6:472 - 83; http://dx.doi.org/10.1016/j.cmet.2007.11.004; PMID: 18054316
- Lansard M, Panserat S, Plagnes-Juan E, Dias K, Seiliez I, Skiba-Cassy S. L-leucine, L-methionine, and L-lysine are involved in the regulation of intermediary metabolism-related gene expression in rainbow trout hepatocytes. J Nutr 2011; 141:75 - 80; http://dx.doi.org/10.3945/jn.110.124511; PMID: 21106925
- Lansard M, Panserat S, Plagnes-Juan E, Seiliez I, Skiba-Cassy S. Integration of insulin and amino acid signals that regulate hepatic metabolism-related gene expression in rainbow trout: role of TOR. Amino Acids 2010; 39:801 - 10; http://dx.doi.org/10.1007/s00726-010-0533-3; PMID: 20213441
- Polakof S, Panserat S, Craig PM, Martyres DJ, Plagnes-Juan E, Savari S, et al. The metabolic consequences of hepatic AMP-kinase phosphorylation in rainbow trout. PLoS ONE 2011; 6:e20228; http://dx.doi.org/10.1371/journal.pone.0020228; PMID: 21625448
- Seiliez I, Gabillard JC, Skiba-Cassy S, Garcia-Serrana D, Gutierrez J, Kaushik S, et al. An in vivo and in vitro assessment of TOR signaling cascade in rainbow trout (Oncorhynchus mykiss). Am J Physiol Regul Integr Comp Physiol 2008; 295:R329 - 35; http://dx.doi.org/10.1152/ajpregu.00146.2008; PMID: 18434442
- Seiliez I, Sabin N, Gabillard JC. FoxO1 Is Not a Key Transcription Factor in the Regulation of myostatin (mstn-1a and mstn-1b) Gene Expression in Trout Myotubes. Am J Physiol Regul Integr Comp Physiol 2011; 301:R97 - 104; http://dx.doi.org/10.1152/ajpregu.00828.2010; PMID: 21490365
- Seiliez I, Panserat S, Skiba-Cassy S, Polakof S. Effect of acute and chronic insulin administrations on major factors involved in the control of muscle protein turnover in rainbow trout (Oncorhynchus mykiss). Gen Comp Endocrinol 2011; 172:363 - 70; http://dx.doi.org/10.1016/j.ygcen.2011.03.026; PMID: 21463630
- Meijer AJ, Codogno P. Signalling and autophagy regulation in health, aging and disease. Mol Aspects Med 2006; 27:411 - 25; http://dx.doi.org/10.1016/j.mam.2006.08.002; PMID: 16973212
- Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell 2010; 140:313 - 26; http://dx.doi.org/10.1016/j.cell.2010.01.028; PMID: 20144757
- Fass E, Shvets E, Degani I, Hirschberg K, Elazar Z. Microtubules support production of starvation-induced autophagosomes but not their targeting and fusion with lysosomes. J Biol Chem 2006; 281:36303 - 16; http://dx.doi.org/10.1074/jbc.M607031200; PMID: 16963441
- Ohsumi Y. Molecular dissection of autophagy: two ubiquitin-like systems. Nat Rev Mol Cell Biol 2001; 2:211 - 6; http://dx.doi.org/10.1038/35056522; PMID: 11265251
- Bruhat A, Cherasse Y, Chaveroux C, Maurin AC, Jousse C, Fafournoux P. Amino acids as regulators of gene expression in mammals: molecular mechanisms. Biofactors 2009; 35:249 - 57; http://dx.doi.org/10.1002/biof.40; PMID: 19415732
- Herningtyas EH, Okimura Y, Handayaningsih AE, Yamamoto D, Maki T, Iida K, et al. Branched-chain amino acids and arginine suppress MaFbx/atrogin-1 mRNA expression via mTOR pathway in C2C12 cell line. Biochim Biophys Acta 2008; 1780:1115-20.
- Tesseraud S, Metayer-Coustard S, Boussaid S, Crochet S, Audouin E, Derouet M, et al. Insulin and amino acid availability regulate atrogin-1 in avian QT6 cells. Biochem Biophys Res Commun 2007; 357:181 - 6; http://dx.doi.org/10.1016/j.bbrc.2007.03.131; PMID: 17418104
- Yecies JL, Manning BD. Transcriptional control of cellular metabolism by mTOR signaling. Cancer Res 2011; 71:2815 - 20; http://dx.doi.org/10.1158/0008-5472.CAN-10-4158; PMID: 21487041
- Boya P, Gonzalez-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, et al. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol 2005; 25:1025 - 40; http://dx.doi.org/10.1128/MCB.25.3.1025-1040.2005; PMID: 15657430
- Mizushima N. Methods for monitoring autophagy. Int J Biochem Cell Biol 2004; 36:2491 - 502; http://dx.doi.org/10.1016/j.biocel.2004.02.005; PMID: 15325587
- Codogno P, Meijer AJ. Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ 2005; 12:1509 - 18; http://dx.doi.org/10.1038/sj.cdd.4401751; PMID: 16247498
- Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat Cell Biol 2010; 12:814 - 22; http://dx.doi.org/10.1038/ncb0910-814; PMID: 20811353
- Avruch J, Long X, Ortiz-Vega S, Rapley J, Papageorgiou A, Dai N. Amino acid regulation of TOR complex 1. Am J Physiol Endocrinol Metab 2009; 296:E592 - 602; http://dx.doi.org/10.1152/ajpendo.90645.2008; PMID: 18765678
- Kimball SR, Jefferson LS. New functions for amino acids: effects on gene transcription and translation. Am J Clin Nutr 2006; 83:500S - 507S; PMID: 16470021
- Jousse C, Averous J, Bruhat A, Carraro V, Mordier S, Fafournoux P. Amino acids as regulators of gene expression: molecular mechanisms. Biochem Biophys Res Commun 2004; 313:447 - 52; http://dx.doi.org/10.1016/j.bbrc.2003.07.020; PMID: 14684183
- Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, et al. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab 2008; 8:224 - 36; http://dx.doi.org/10.1016/j.cmet.2008.07.007; PMID: 18762023
- Mizushima N. Autophagy: process and function. Genes Dev 2007; 21:2861 - 73; http://dx.doi.org/10.1101/gad.1599207; PMID: 18006683
- Johnston IA, Bower NI, Macqueen DJ. Growth and the regulation of myotomal muscle mass in teleost fish. J Exp Biol 2011; 214:1617 - 28; http://dx.doi.org/10.1242/jeb.038620; PMID: 21525308
- National-Research-Council, ed. Nutrient requirements of fish. Washington, DC, USA, 1993.
- Mehrpour M, Esclatine A, Beau I, Codogno P. Overview of macroautophagy regulation in mammalian cells. Cell Res 2010; 20:748 - 62; http://dx.doi.org/10.1038/cr.2010.82; PMID: 20548331
- Gabillard JC, Sabin N, Paboeuf G. In vitro characterization of proliferation and differentiation of trout satellite cells. Cell Tissue Res 2010; 342:471 - 7; http://dx.doi.org/10.1007/s00441-010-1071-8; PMID: 21086139
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001; 29:e45; http://dx.doi.org/10.1093/nar/29.9.e45; PMID: 11328886
- Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 2002; 30:e36; http://dx.doi.org/10.1093/nar/30.9.e36; PMID: 11972351
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72:248 - 54; http://dx.doi.org/10.1016/0003-2697(76)90527-3; PMID: 942051
- SIGENAE. Information System of Breeding Animals' Genome. http://www.sigenae.org