Abstract
The accumulation of α-synuclein is critical for the development of Parkinson disease (PD), and unraveling the mechanisms that regulate α-synuclein levels is key to understanding the pathophysiology of the disease. We recently found that USP9X deubiquitinates α-synuclein, and that this process determines the partition of α-synuclein between the proteasomal and autophagy pathways. By manipulating USP9X levels, we observed that monoubiquitinated α-synuclein is degraded by the proteasome, whereas deubiquitination of α-synuclein favors its degradation by autophagy. As USP9X levels and activity are decreased in α-synucleinopathy brains, USP9X may now represent a novel target for PD.
PD is a neurodegenerative disease that leads to motor impairment and that decreases the quality of life. Even though the accumulation of α-synuclein is accredited as central to the pathogenesis of PD, much about the molecular mechanisms that trigger the disease still needs to be uncovered.
The accumulation of α-synuclein leads to neuronal toxicity and is considered one of the starting points of the disease. Once accumulated, α-synuclein aggregates and promotes inclusion formation, which eventually culminates in the formation of Lewy bodies. The accumulation of oligomeric α-synuclein is thought to be cytotoxic, while the role of more complex α-synuclein aggregates is still arguable. One possible strategy to treat the disease is to decrease soluble α-synuclein levels in order to avoid its accumulation rather than to promote the dissociation of protein aggregates.
All major known degradation pathways have been implicated in the degradation of α-synuclein. On the one hand, macroautophagy (referred to here as autophagy) is considered to play a key role in α-synuclein degradation because the autophagy inhibitor 3-methyladenine promotes a robust increase in the levels of α-synuclein. On the other hand, α-synuclein is also degraded by the proteasome.
What are the mechanisms regulating α-synuclein partition between the autophagy and proteasomal pathways? Our recent study describing the role of α-synuclein monoubiquitination is a step closer for understanding the relative roles of the different degradation pathways in α-synuclein biology or pathology (). We have previously shown that α-synuclein is monoubiquitinated by SIAH, and that the lysines ubiquitinated by SIAH are the same ones ubiquitinated in α-synuclein purified from Lewy bodies. Also, inhibition of proteolysis leads to the accumulation of monoubiquitinated α-synuclein and to the formation of toxic inclusions, indicating that α-synuclein inclusions may contribute to the triggering and/or progression of the disease.
Figure 1. Monoubiquitination determines α-synuclein fate. USP9X deubiquitinates α-synuclein, and nonubiquitinated α-synuclein is mostly degraded by the autophagy pathway. Autophagy impairment may favor α-synuclein inclusion formation. SIAH monoubiquitinates α-synuclein, which leads to its degradation by the proteasome. Cytosolic USP9X is decreased in α-synucleinopathies and this will contribute to the accumulation of monoubiquitinated α-synuclein. Since monoubiquitinated α-synuclein is more prone to aggregation, it may contribute to Lewy body formation caused by proteasomal/autophagy dysfunction. Although monoubiquitination of α-synuclein promotes the formation of toxic inclusions, it is still not clear if Lewy bodies are cytotoxic or cytoprotective.
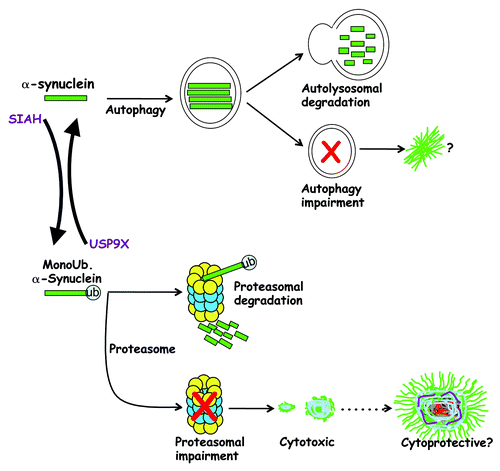
We recently found that the monoubiquitination of α-synuclein is a dynamic process, and not only a secondary event subsequent to α-synuclein aggregation (). Monoubiquitination of α-synuclein determines its partition between the autophagy and proteasomal pathways. This process is regulated by USP9X, which is an endogenous α-synuclein deubiquitinase that removes monoubiquitin chains. When USP9X levels are suppressed, α-synuclein monoubiquitination increases, leading to a decrease in the steady-state levels of α-synuclein. Degradation of monoubiquitinated α-synuclein is prevented solely by proteasome inhibitors, indicating that mononoubiquitination targets α-synuclein for proteasomal degradation (). Similarly, autophagy inhibition has little effect on the degradation of monoubiquitinated α-synuclein. The opposite is observed when α-synuclein is deubiquitinated by overexpressing USP9X, in which α-synuclein levels drastically increase in the presence of autophagy inhibitors alone. Thus, while monoubiquitinated α-synuclein is targeted for degradation by the proteasomal system, deubiquitinated α-synuclein is preferentially degraded by autophagy ().
In agreement with the in vivo experiments, monoubiquitinated, but not deubiquitinated, α-synuclein is degraded by purified 26S proteasomes. This finding is unique since, usually, the attachment of an ubiquitin tree of at least four ubiquitin molecules is required to promote degradation by the proteasome. Nevertheless, a few reports have shown that monoubiquitination and multi-monoubiquitination can be enough to engage specific proteins to the proteasome. In the case of α-synuclein, multi-monoubiquitination is likely to be responsible for targeting it to the proteasome since we have previously shown by mass spectrometry analysis the presence of different α-synuclein lysine residues being simultaneously monoubiquitinated by SIAH. Accordingly, in vivo and in vitro experiments, performed in conditions favoring monoubiquitination, point to a more important contribution of the proteasome in degrading α-synuclein than previously anticipated. They also highlight the distinctive role of monoubiquitination in this process.
Several studies suggest that different degradation pathways might be compromised in PD. Thus, we also investigated the role of α-synuclein monoubiquitination under conditions of proteolytic impairment thought to occur in the disease. We observed that the accumulation of α-synuclein monoubiquitination by USP9X knockdown leads to a marked increase in the formation of toxic α-synuclein inclusions (), which further confirms the strong aggregation-prone trait added to α-synuclein once it is monoubiquitinated and not properly degraded.
Is USP9X-regulated α-synuclein monoubiqutination dysfunctional in α-synucleinopathies? To answer this question, we monitored USP9X levels and activity in PD and Diffuse Lewy Body disease (DLBD). USP9X levels are drastically decreased in the soluble fractions of tissues from PD and DLBD, and this is correlated with lower deubiquitinase activity toward α-synuclein in tissue extracts from patients. USP9X accumulates in Lewy bodies and Lewy neurites of both PD and DLBD patients, where it is likely to be inactive due to the high degree of protein aggregation in these structures. These data suggest that the lower cytosolic USP9X levels, in combination with proteolytic dysfunction, might contribute to α-synuclein aggregation and Lewy body formation in the disease (). Since α-synuclein inclusions, in conditions of increased monoubiquitination, are toxic to cells, it is possible that Lewy bodies may be deleterious to cells during their initial formation stages. The toxicity of more mature Lewy bodies is less clear since the recruitment of PD-related proteins, such as synphilin-1 isoforms, provides a more benign feature to α-synuclein inclusions ().
In summary, our recent study indicates that autophagy and proteasomal degradation target different α-synuclein species. The decrease of USP9X levels in α-synucleinopathy brains will contribute to α-synuclein monoubiquitination and accumulation in PD. In this context, drugs acting on USP9X might represent a novel strategy for therapeutic intervention in combination with proteasome and autophagy activators.