Abstract
Maternal inheritance of mitochondrial DNA (mtDNA) is generally observed in many eukaryotes. Sperm-derived paternal mitochondria and their mtDNA enter the oocyte cytoplasm upon fertilization and then normally disappear during early embryogenesis. However, the mechanism underlying this clearance of paternal mitochondria has remained largely unknown. Recently, we showed that autophagy is required for the elimination of paternal mitochondria in Caenorhabditis elegans embryos. Shortly after fertilization, autophagosomes are induced locally around the penetrated sperm components. These autophagosomes engulf paternal mitochondria, resulting in their lysosomal degradation during early embryogenesis. In autophagy-defective zygotes, paternal mitochondria and their genomes remain even in the larval stage. Therefore, maternal inheritance of mtDNA is accomplished by autophagic degradation of paternal mitochondria. We also found that another kind of sperm-derived structure, called the membranous organelle, is degraded by zygotic autophagy as well. We thus propose to term this allogeneic (nonself) organelle autophagy as allophagy.
Mitochondria are essential organelles that are responsible for energy production and various signaling pathways, and they have their own DNA. Oocytes and sperm have their own mitochondria and mtDNA, and the zygote receives both maternal and paternal mtDNA after fertilization. Despite this, in most species including humans, the mtDNA is inherited solely from the mitochondria of the original oocytes. This pattern of inheritance, referred to as “maternal inheritance of mtDNA,” has been a dogma in human evolutionary studies. However, it remains largely unknown how sperm-derived paternal mitochondria are selectively removed from the cytoplasm of the zygote during embryogenesis, although involvement of the ubiquitin-proteasome pathway has been suggested in mammals. Recently, we reported for the first time that autophagy is required for selective degradation of paternal mitochondria in C. elegans embryos.
C. elegans sperm lack a flagellum (midpiece and tail structures) but contain 50–70 tightly packed mitochondria around the nucleus in the cell body (). We fluorescently marked sperm mitochondria with MitoTracker or mitochondria-targeted GFP, and observed their fate in the fertilized embryos. Similar to the mammalian embryos, sperm mitochondria enter the oocyte cytoplasm upon fertilization but gradually disappear by the 16-cell stage. We found that immediately after fertilization, autophagosomes visualized by LGG-1 (the worm Atg8/LC3 homolog) are formed locally around the penetrated sperm components and sequester the paternal mitochondria. Among the entire mitochondrial population, autophagosomes seemed to selectively engulf paternal mitochondria. Engulfment of paternal mitochondria by autophagosomes results in lysosomal degradation during early embryogenesis. When the lgg-1 gene is knocked out, paternal mitochondria and their genome persist in the late-stage embryos and even in the first larval stage. These results demonstrate that autophagy is required for the clearance of paternal mitochondria and their mtDNA from zygotic cytoplasm.
Figure 1. Fertilization-triggered autophagy degrades sperm-derived mitochondria and membranous organelles (MOs). Sperm MOs are specialized vesicular structures essential for sperm fertility. Sperm mitochondria and MOs enter the oocyte cytoplasm upon fertilization. Shortly after fertilization, ubiquitin (Ub)-signals are detected on MOs but not on the paternal mitochondria. Then sperm-derived mitochondria and MOs are sequestered by newly formed autophagosomes and degraded in lysosomes during early embryogenesis. This results in maternal inheritance of mitochondria and mtDNA. n, pronuclei.
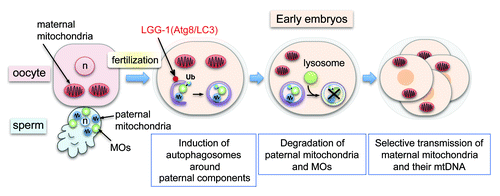
The question then is how do autophagosomes specifically recognize paternal mitochondria? In mammals, sperm mitochondria seem to be tagged by ubiquitin before fertilization. Involvement of ubiquitination has been also reported in mitophagy in somatic cells. In C. elegans, however, no obvious ubiquitin signal is detected on sperm mitochondria before or after fertilization. Instead, strong ubiquitin signals are detected on another kind of sperm-originated structure, membranous organelles (MOs), in fertilized embryos. MOs are specialized ER-Golgi-derived vesicles essential for sperm fertility and are the major membrane-bound structure in mature sperm apart from the mitochondria. Ubiquitinated MOs are also engulfed by autophagosomes, suggesting that paternally derived organelles are generally eliminated from embryos by autophagy. Paternal mitochondria can be engulfed by autophagosomes separately from MOs, although some autophagosomes contain both. This observation implies that paternal mitochondria are recognized independent of ubiquitination on MOs. Thus, we have no direct evidence for the involvement of ubiquitination in the clearance of paternal mitochondria in worms. However, it is still possible that low-level ubiquitination, undetectable by immunostaining, is involved. We also realized that paternal mitochondria possess a characteristic granular morphology that is quite different from that of typical mitochondria. Paternal mitochondria also seem to be proliferation- and fusion-inactive, because those inherited in the lgg-1 mutant retain the granular morphology and do not increase in number during embryogenesis. Such qualitative differences may underlie selective recognition of paternal mitochondria. In other words, before fertilization, sperm mitochondria may already be programmed for rapid degradation in embryos.
We also observed that induction of autophagy in zygotes is very local and strongly linked to fertilization, i.e., sperm entry. Moreover, in polyspermic embryos, autophagosomes are induced around each penetrated sperm. These observations imply that recognition of penetrated sperm components leads to localized autophagosome formation. In this regard, zygotic autophagy of paternal organelles is reminiscent of autophagy induced by invading bacteria in somatic cells. Autophagosome formation after fertilization is regulated by the canonical Atg pathway including UNC-51 (C. elegans Atg1/ULK1 homolog) and ATG-7. How sperm entry signals to this Atg pathway is an interesting point to be addressed.
Another important question is why paternal mitochondria and mtDNA should be eliminated from embryos. One hypothesis is that the paternal mitochondria and/or mtDNA are heavily damaged by reactive oxygen species prior to fertilization, and are hence removed to prevent the spread of potentially deleterious mitochondria to the whole population. Alternatively, the heteroplasmic state of mtDNA might have some unfavorable effects on survival. Knockout of lgg-1 causes embryonic or first-stage larval lethality, indicating that autophagic activity is essential for normal development. However, this lethality could be due to other effects of impaired autophagy.
To answer these questions, we need to identify factors specifically involved in paternal mitochondria degradation and characterize the phenotypes of mutants of such factors.