Abstract
Toxoplasma gondii belongs to the phylum Apicomplexa, a diverse group of early branching unicellular eukaryotes related to dinoflagellates and ciliates. Like several other Apicomplexa such as Plasmodium (the causative agent of malaria), T. gondii is a human pathogen responsible for a potentially lethal disease called toxoplasmosis. Most Apicomplexa have complex life cycles, involving intermediate hosts and vectors, which include obligatory intracellular developmental stages. In the case of malaria and toxoplasmosis, it is that replicative process, leading to the ultimate lysis of the host cell, which is causing the symptoms of the disease. For Toxoplasma, the invasive and fast-replicating form of the parasite is called the tachyzoite. While autophagy has been a fast-growing field of research in recent years, not much was known about the relevance of this catabolic process in medically important apicomplexan parasites. Vesicles resembling autophagosomes had been described in drug-treated Plasmodium parasites in the early 1970s and a potential role for autophagy in organelle recycling during differentiation between Plasmodium life stages has also been recently described. Interestingly, recent database searches have identified putative orthologs of the core machinery responsible for the formation of autophagosomes in several protists, including Toxoplasma. In spite of an apparently reduced machinery (only about one-third of the yeast ATG genes appear to be conserved), T. gondii seemed thus able to perform macroautophagy, but the cellular functions of the pathway for this parasite remained to be demonstrated.
We have recently published the first experimental demonstration of a functional autophagic pathway in T. gondii. In order to detect and quantify autophagosomes in T. gondii tachyzoites, we have used TgATG8 (the ortholog of yeast Atg8) as a marker. Using a specific anti-TgATG8 antibody and a GFP-TgATG8-overexpressing transgenic T. gondii cell line, we could describe autophagosome formation in tachyzoites following amino acid starvation, a well-known inducer of autophagy. In our assay, free tachyzoites (allowing a more direct control of environmental conditions and nutrient access, compared with intracellular parasites) were exposed to a starvation medium for increased periods of time.
We gathered several lines of evidence of starvation-induced autophagy in Toxoplasma. First, by fluorescence microscopy, we could see a relocalization of TgATG8 from the cytoplasm of the tachyzoites to vesicular structures. Second, after starvation, by immuno-electron microscopy, we observed vesicles bearing the hallmarks of autophagosomes and autolysosomes. Third, western blot analysis following urea SDS-PAGE allowed the separation of soluble TgATG8 from its lipid-conjugated form (the phagophore and autophagosome-bound form) and the latter increases with starvation.
Using a TgATG8-based quantification assay, we could test the effects of inhibitors of upstream regulators including Tor and PtdIns 3-kinase on the modulation of autophagy. However, high concentrations of drugs were needed to see a significant effect, which precluded the use of these inhibitors (also active on host cells) to precisely investigate autophagic function in intracellular parasites.
Toxoplasma tachyzoites divide by a process called endodyogeny, by which two daughter cells are growing inside a mother cell (). During this process, some organelles are duplicated, while others, like the apical organelles, are synthesized de novo. Our initial hypothesis was that autophagy might be used by these parasites to recycle maternal organelles. During the cell cycle of the parasites, autophagosomes are detected right after invasion of the host cell, but soon disappeared. This could mean that autophagy might be used to provide a nutrient source before the tachyzoite establishes itself in its host (as it invades its host cell, Toxoplasma builds a parasitophorous vacuole and gets relatively quick access to nutrient sources from the host). Yet, autophagosomes were also seen transiently in long-established parasites, almost systematically in dividing parasites, suggesting a role for autophagy during division of the tachyzoites.
Figure 1. Schematic representation of the T. gondii lytic cycle. Squared text indicates different steps in the cycle. Text in black boxes describes putative roles for autophagy during distinctive phases of the cycle. The inset describes the mode of parasite replication called endodyogeny, with two daughter parasites developing inside a mother cell. During this process, some organelles are synthesiszed de novo (i.e., apical organelles), while others are replicated (i.e., nucleus, mitochondrion).
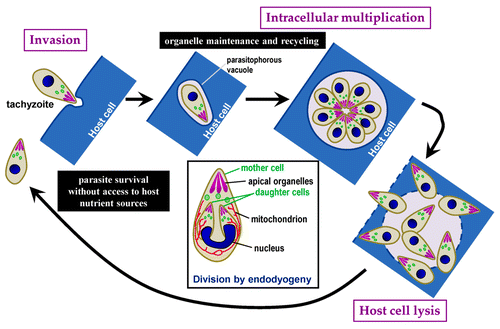
We sought to produce an autophagy-deficient mutant by targeting the T. gondii ATG3 ortholog. ATG3 is, in other eukaryotes, essential for the association of ATG8 with the phagophore membrane, and is thus crucial for autophagic function. We produced a conditional TgATG3 null mutant, allowing us to specifically control the depletion of TgATG3. TgATG8 lipidation, and its autophagosome association, were seriously affected in TgATG3-depleted parasites, confirming that autophagic function was likely to be impaired. These parasites were also profoundly altered in growth and intracellular development. However, to our surprise, no accumulation of organelles or morphological alteration of de novo synthesized organelles was observed in the mutant parasites, suggesting that TgATG3-dependent autophagy is not extensively required for recycling of mother organelles during parasite division. Nevertheless, a striking cellular phenotype of TgATG3 depletion is the structural and functional alteration of the mitochondrial network in the parasites. In tachyzoites, the mitochondrion is usually present as a single reticulated network during the whole duration of the cell cycle. However, mutant parasites display a fragmented mitochondrion, and mitochondrial material is expelled into the parasitophorous vacuole.
T. gondii tachyzoites have a diminished capacity to remain virulent the longer the parasites are deprived of host cells and we have shown that autophagy is potentially triggered by starvation in extracellular tachyzoites. Whether this is physiologically relevant to situations that can be encountered in the host, and autophagy can increase the chances of parasite survival in this context is unknown at the moment. However, as a housekeeping function, autophagy might be involved in maintaining parasite mitochondrial homeostasis by quality control of parts of the network during the intracellular developmental cycle. There is increasing evidence of a mitochondria-autophagy crosstalk in eukaryotes, and the results we have obtained in T. gondii further illustrate this. We have clues of mitochondrion-specific autophagy (mitophagy) in Toxoplasma, but the molecular machinery for specific mitophagy is currently unknown in this parasite. If anything, this illustrates that many questions concerning the autophagic process remain to be solved in Toxoplasma.
There is a fundamental interest in studying an early branching eukaryote, with an apparently reduced machinery, to provide information on autophagy as a mechanism. Beyond this, we have validated autophagy as an essential pathway for the growth of this pathogen and we can now consider identifying targets that could be interfered with to block this cellular function.