Abstract
In MCF-7 breast tumor cells, ionizing radiation promoted autophagy that was cytoprotective; pharmacological or genetic interference with autophagy induced by radiation resulted in growth suppression and/or cell killing (primarily by apoptosis). The hormonally active form of vitamin D, 1,25D3, also promoted autophagy in irradiated MCF-7 cells, sensitized the cells to radiation and suppressed the proliferative recovery that occurs after radiation alone. 1,25D3 enhanced radiosensitivity and promoted autophagy in MCF-7 cells that overexpress Her-2/neu as well as in p53 mutant Hs578t breast tumor cells. In contrast, 1,25D3 failed to alter radiosensitivity or promote autophagy in the BT474 breast tumor cell line with low-level expression of the vitamin D receptor. Enhancement of MCF-7 cell sensitivity to radiation by 1,25D3 was not attenuated by a genetic block to autophagy due largely to the promotion of apoptosis via the collateral suppression of protective autophagy. However, MCF-7 cells were protected from the combination of 1,25D3 with radiation using a concentration of chloroquine that produced minimal sensitization to radiation alone. The current studies are consistent with the premise that while autophagy mediates a cytoprotective function in irradiated breast tumor cells, promotion of autophagy can also confer radiosensitivity by vitamin D (1,25D3). As both cytoprotective and cytotoxic autophagy can apparently be expressed in the same experimental system in response to radiation, this type of model could be utilized to distinguish biochemical, molecular and/or functional differences in these dual functions of autophagy.
Introduction
While chemotherapy and radiation treatment generally demonstrate initial effectiveness in suppressing breast cancer growth and eliminating the vast majority of the breast tumor cell population, patients nevertheless may succumb to the disease upon its recurrence either in the breast tissue or as metastatic lesions at other organ sites such as the bones and brain.Citation1 Although strategies have been developed in efforts to enhance the response to chemotherapy and radiation by interfering with cytoprotective signaling, there have been few direct attempts to address the problem of disease recurrence. One promising approach based on our previous workCitation2-Citation5 may be to utilize vitamin D3 or its analogs in combination with conventional therapies.
1,25D3, a steroid hormone with a central role in regulating calcium homeostasis, has been shown to produce pronounced effects on cell growth, differentiation and survival.Citation6-Citation8 Because of the possibility of hypercalcemia with the utilization of 1,25D3 at high doses,Citation9 analogs of 1,25D3 such as EB1089 have been developed that demonstrate similar growth regulatory effects, while having a reduced impact on calcium metabolism.Citation6,Citation10 Additionally, vitamin D3 (cholecalciferol), and the active form 1,25 dihydroxyvitamin D3 (1,25D3) have been used experimentally in combination with a number of established treatments in preclinical cancer studies.Citation11-Citation16
MCF-7 cells, a commonly used p53 wild-type, caspase-3 deficient breast tumor cell line, have proven to be relatively refractory to apoptosis in response to ionizing radiation when radiation (IR) was administered either as a single dose of 10 Gy or more clinically relevant fractionated doses of 5×2 Gy, instead undergoing a form of senescence arrest.Citation2,Citation17 In contrast, treatment with the vitamin D analogs, EB1089 or ILX-23-7553, prior to radiation was found to change the response to irradiation from growth arrest to cell death.Citation2-Citation5 Our studies further excluded apoptosis and mitotic catastrophe as the modes of cell death when EB1089 was combined with radiation and were consistent with cell death occurring through autophagy.Citation2,Citation3
Autophagy reflects a cellular response to stress in which the fusion of autophagosomes and lysosomes allows the degradation of subcellular organelles to generate energy and metabolic precursors; autophagy may be cytoprotective or cytotoxic, depending on the cells and the nature of the stressful challenge.Citation18-Citation21 Our current studies were designed to evaluate the involvement of autophagy in radiosensitization by EB1089 as well as extending the work to the hormonally active and natural form of 1,25D3. In addition, studies were performed in breast tumor cells that are intrinsically resistant to radiation and chemotherapy through overexpression of Her-2/neu (MCF7/Her-2/neu cells),Citation22 in Hs578t cells, another (moderate Her-2/neu overexpression, p53 mutant) breast tumor cell line that was shown to be radiosensitized by 1,25D3,Citation23,Citation24 as well as p53 mutant, Her-2 overexpressing BT474 cells, a breast tumor cell line that has been reported to express the vitamin D receptor.Citation25
Results
Promotion of autophagy in cells exposed to EB1089 and ionizing radiation
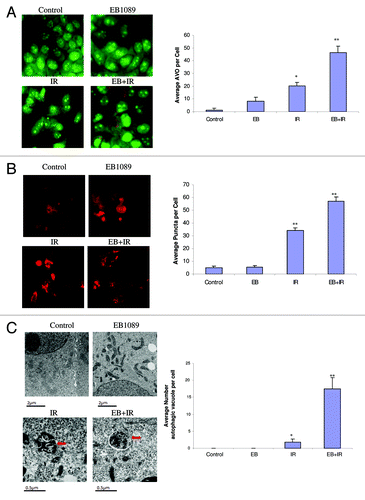
In previous studies, we proposed that the impact of the combination of the vitamin D analog, EB1089, with radiation on cell survival was primarily through the promotion of autophagy.Citation3 In the current studies, the promotion of autophagy in MCF-7 cells exposed to EB1089 + radiation was confirmed based on acridine orange staining of autophagosomesCitation26 (), redistribution of RFP-LC3 indicative of LC3 association with the autophagosomal membraneCitation27 () and evidence of autophagic vacuole formation by electron microscopyCitation18-Citation21 (). Quantification for each study is shown in an accompanying bar graph. Acridine orange vesicle staining in MCF-7 cells exposed to tamoxifen was utilized as a positive control for autophagyCitation28(data not shown). For all three assays, untreated control cells or cells treated with EB1089 alone show minimal or no evidence for autophagy while cells treated with radiation alone, or in combination with EB1089, exhibit a significant increase over that observed in controls (albeit to different degrees for the three assays).
Figure 1. Promotion of autophagy by EB1089 + radiation in MCF-7 cells. (A) Acridine orange staining of autophagic vesicles. Cells were exposed to 100 nM EB1089 for 72 h alone, radiation alone (5×2 Gy administered over a period of 3 d) or EB1089 for 72 h, which was then removed and immediately followed by 5×2 Gy administered over a period of 3 d. AO images were taken 24 h post-irradiation using an inverted fluorescence microscope. Average number of AVOs per cell were counted in three fields for each condition and are represented in the right panel graph. (B) RFP LC3 punctate staining. MCF-7 cells stably transfected with RFP-LC3 were treated as in A and diffuse and punctate staining was monitored by fluorescence microscopy. Again, images were taken 24 h post-irradiation. Average number of puncta per cell were counted in three fields for each condition and are represented in the right panel graph. (C) Electron microscopy of autophagic vesicles. MCF-7 cells were treated as in (A) fixed and subjected to electron microscopy. Autophagic vacuoles are indicated by the arrows. Scale bars indicate magnification. Average numbers of autophagic vacuoles per cell were counted and are represented in the right panel graph.
Promotion of autophagy by 1,25D3 and radiation in MCF7 breast tumor cells
Although analogs of vitamin D3 such as EB1089 have been generated with the goal of developing agents that express the activity of the parent compound but with reduced calcemic activity,Citation29 the utility of administering 1,25D3, the hormonally active form of vitamin D, with minimal and acceptable levels of toxicity to patients, has been established.Citation30 Consequently, studies were performed to determine whether 1,25D3 produced the same impact as EB1089 on radiation sensitivity through the promotion of autophagy and interference with proliferative recovery. presents the results of experiments where MCF-7 cells were exposed to 5×2 Gy radiation alone or with 100 nM 1,25D3 prior to irradiation and where viable cell number was monitored over an extended interval in order to determine whether the residual surviving cells maintained a prolonged growth arrested state. The 1,25D3 inhibits growth, but tumor cell growth is restored once 1,25D3 is removed from the experimental system. Radiation produces a transient growth arrest that was previously shown to reflect senescence;Citation2,Citation3,Citation17 of particular note is the fact that the cells recover proliferative capacity within a period of 5–7 d. As in our previous studies with EB1089,Citation2,Citation3 prior exposure to 1,25D3 produces a more pronounced and prolonged growth arrest with subsequent cell death; furthermore, the exposure to 1,25D3 suppresses the proliferative recovery that is evident with radiation alone. Overall, by day 12 there is an approximate 84% reduction in cell number for the combination treatment compared with radiation alone, which is consistent with our previously reported studies combining EB1089 with radiation.Citation2
Figure 2. Influence of 1,25 D3 on the response to fractionated radiation in MCF-7 cells. MCF-7 cells were exposed to radiation alone (5×2 Gy), or 100 nM 1,25D3 for 72 h; cells were washed free of the 1,25D3 prior to irradiation (5×2 Gy). (A) Viable cell number was determined by exclusion of trypan blue at the indicated days following the initiation of radiation exposure. *p < 0.05 and **p < 0.0001 from IR alone on corresponding day. ↑ Indicate days when irradiation occurred. (B) Clonogenic survival was assessed after 14 d post-treatment. Values shown are from a representative experiment with triplicate samples for each condition. *p < 0.05 and **p < 0.0001 from control. (C) Autophagy was monitored based on acridine orange staining 24 h post-irradiation. (D) Electron microscopic imaging of autophagy; arrowheads indicates autophagic vacuoles. Scale bars indicate magnification. For 1,25D3 + radiation, 1 µm magnification is shown to more clearly demonstrate autophagic vacuole formation. (E) Autophagic flux was based on the decline in p62 levels monitored by western blotting 24 h post-irradiation. Actin was utilized as a loading control. Serum starvation was used as a positive control for autophagic flux. The accompanying bar graph presents quantification of the data.
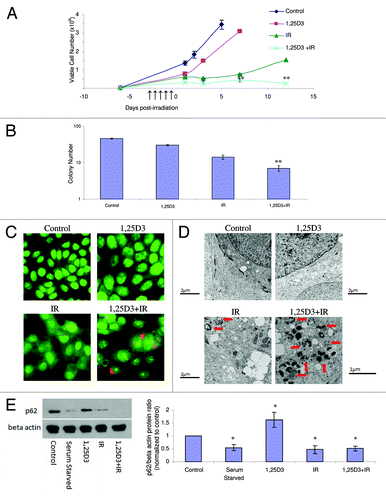
In addition to cell viability studies, the impact of the treatment combining 1,25D3 with ionizing radiation on clonogenic survival is demonstrated in and Figure S1A. While radiation alone significantly reduces colony formation (74%) in comparison to control cells, 1,25D3 in combination with radiation induces an 87% reduction in colony formation () and a 73% reduction in colony size (Fig. S1A). As explained previously,Citation2 the clonogenic survival assay is likely to under-represent the impact of the combination treatment as compared with radiation alone since the proliferative recovery that is evident in likely does not occur under the experimental conditions of the clonogenic survival assay because of the lack of cell to cell contact; consequently, the differences between radiation alone and the combination of 1,25D3 + radiation will not be as pronounced as for the studies presented in .
To determine if the combination of 1,25D3 with radiation increased levels of autophagy, acridine orange, RFP-LC3 punctate, and electron microscopy images were monitored and quantified. indicates that, based on acridine orange staining, virtually no autophagy was detectable with either control cells or cells treated with 1,25D3 alone, while radiation and the combination increased acridine orange staining, indicative of autophagy. Acridine orange quantification has been provided in Figure S1B. In Figure S1C (left and right panels), MCF-7 RFP-tagged LC3 cells demonstrate a significant increase of red punctate fluorescence with radiation alone as well as with 1,25D3 followed by radiation, indicative of LC3 association with the autophagosomal membrane.Citation27 In contrast, a diffuse pattern of staining is observed in controls and in cells treated with 1,25D3 alone. The electron microscopy images in (with quantification of autophagic vesicles in Fig. S1D) further confirms the substantial induction of autophagy with the combination of 1,25D3 + radiation (as shown by the presence of autophagic vesicles indicated with arrowheads), with detectable but significantly lesser number of autophagosomes detected with radiation alone.
It is now recognized that although autophagosome formation is a necessary component of the autophagic process, autophagosome formation can occur without completion of autophagy and degradation of the autophagosomal content; consequently, it becomes necessary to also assess autophagic flux.Citation31 To evaluate autophagic flux, levels of the p62 protein were monitored by western blotting ( and accompanying bar graph). As a positive control, a decline in p62 levels was evident when the cells were serum starved. Treatment with 1,25D3 alone did not reduce and in fact appeared to moderately increase p62 levels. The combination of 1,25D3 and radiation clearly promoted robust autophagic flux, as indicated by the decline in p62 levels. Furthermore, radiation alone promoted a decline in p62 levels.Footnote* While autophagic flux by radiation alone is generally consistent with the data generated in other assays, the fact that suppression of p62 was similar for radiation alone and the combination of 1,25D3 with radiation was unexpected. However, as p62 function is not limited solely to autophagy but is also associated with ubiquitin mediated protein degradationCitation31-Citation33 it remains possible that our findings may reflect alternative roles of p62 in this system.
Impact of autophagy inhibition on sensitivity to treatment
Given the ongoing controversy as to the differential impact of autophagy in various experimental systems, where autophagy is either cytoprotective or putatively the mode of cell death,Citation18-Citation20,Citation34 studies were designed to examine how interference with autophagy would influence sensitivity to radiation alone as well as treatment with 1,25D3 + radiation. Three commonly used inhibitors of autophagy were initially evaluated both alone and in combination with tamoxifen (data not shown). These included 3-methyladenine (10 mM), which is a PtdIns 3-kinase inhibitor,Citation35 chloroquine (CQ) (25 µM), a weak base that prevents acidification and fusion between the autophagosome and the lysosome,Citation36 and bafilomycin A1 (100 nM), which is an inhibitor of the vacuolar-type H+-ATPase (V-ATPase).Citation37 Due in large part to the toxicity of 3-methyladenine and bafilomycin A1 (or their vehicles), chloroquine was chosen as the pharmacological inhibitor of autophagy in our experimental system. indicates that exposure to CQ alone produced an increase in formation of autophagosomal vesicles, consistent with the weak base’s ability to prevent the completion of autophagy by interfering with acidification. Essentially identical images were generated in studies by Zhao et al. using chloroquine as an autophagy inhibitor in MDA-MB231 cells.Citation38 confirms that chloroquine interfered with the acidification step of autophagy in response to treatment with radiation alone or 1,25D3 + radiation, based on acridine orange staining; interference with tamoxifen-induced autophagy was used as a positive control (data not shown). Furthermore, the autophagic flux induced by radiation or 1,25D3 + radiation is eliminated in the presence of chloroquine; evidence of this blockade to p62 degradation is presented in the p62 western blot shown in with the accompanying bar graph. Levels of p62 were also markedly elevated for the combined treatment of chloroquine, 1,25D3 and radiation.
Figure 3. Effect of autophagy inhibition on the response to radiation or 1,25D3 + radiation. (A) MCF-7 cells were treated with radiation alone (5×2 Gy) or with 1,25D3 + radiation with or without concurrent exposure to 25 µM CQ. Autophagy was monitored by acridine orange staining 24 h post-irradiation. (B) Autophagic flux was based on the decline in p62 levels monitored by western blotting 24 h post-irradiation. Actin was utilized as a loading control. Serum starvation was used as a positive control for autophagic flux. Densitometry for the p62/β actin protein ratio was normalized to controls, **p < 0.0001 from control band. (C) Viable cell number was determined by trypan blue exclusion on the indicated days following treatment. ↑ indicates days on which irradiation was performed. (D) Effect of autophagy inhibition on promotion of apoptosis. MCF-7 cells were treated with IR(5×2 Gy) or with 1,25D3 + radiation with or without concurrent treatment of 25 µM CQ. Apoptosis was detected by the TUNEL assay and DAPI staining 3 d post-irradiation. Taxol treatment was used as a positive control for apoptosis (not shown).
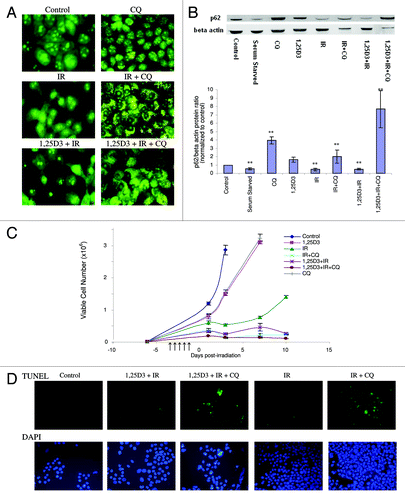
The next series of studies evaluated the temporal response of MCF-7 cells to radiation or 1,25D3 + radiation when autophagy was suppressed by chloroquine. indicates that exposure to either 1,25D3 or CQ alone resulted in only a modest and transient inhibition of growth. As observed in our previous studies, cells treated with radiation alone undergo transient arrest that is followed by recovery of proliferative capacity. Conversely, cells treated with 1,25D3 + radiation decline in cell number and fail to regain proliferative capacity. Furthermore, when autophagy was suppressed by chloroquine in cells exposed to radiation alone, viable cell number declined and recovery of proliferative capacity was abrogated. As shown in , the radiation treatment in cells where autophagy was compromised by chloroquine resulted in extensive apoptosis (TUNEL positive cells co-stained with DAPI to confirm altered nuclear morphology) whereas apoptosis was insignificant with radiation alone (chloroquine treatment alone did not induce apoptosis, not shown). Quantification of TUNEL positive cells at days 1 and 5 is provided in Figure S2.
As a consequence of the observed sensitization to radiation through the suppression of cytoprotective autophagy by CQ (), it was unlikely that CQ treatment would interfere with the effectiveness of the 1,25D3 + radiation combination treatment. In fact, as shown in , the 1,25D3 + radiation combination treatment had an essentially similar impact on viable cell number and promotion of apoptosis as radiation + CQ. While these observations are consistent with studies indicating that autophagy has a cytoprotective action in irradiated cells, as previously suggested in studies by Apel et al. and othersCitation39-Citation41 this created technical difficulties in efforts to demonstrate that autophagy induced by 1,25D3 + radiation is cytotoxic; more specifically a blockade of cytoprotective autophagy would sensitize the cells to radiation. This is, in fact, what occurred in the subsequent genetic silencing studies presented in the following section.
Impact of silencing ATG related genes on susceptibility to 1,25D3 and irradiation
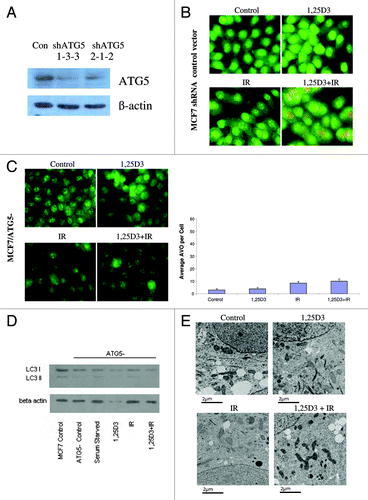
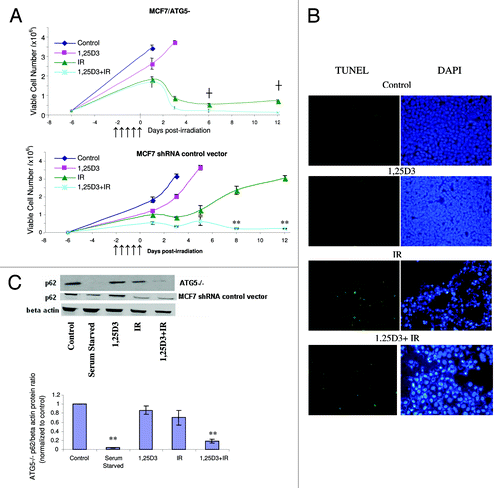
To extend the findings generated by pharmacological inhibition of autophagy, we developed an MCF-7 cell line (MCF-7/ATG5−) in which ATG5, one of the critical autophagy-related genes,Citation42 was silenced. The western blot in indicates a significant reduction in the signal for ATG5 in these cells, although ATG5 expression was not completely silenced. Acridine orange staining in (and quantification in the bar graph in the lower portion of ) confirmed that the response of MCF-7 vector control cells was similar to that of the parental MCF-7 cells in . Acridine orange staining in indicates that the ATG5 knockdown cells exhibited relatively modest intrinsic levels of acridine orange staining in the treatments with 1,25D3, radiation or 1,25D3 + radiation; however, none of these treatments significantly increased orange staining over that in untreated cells. Additionally, the western blot in confirms that the ATG5 knockdown cells had minimal LC3-II accumulation with any of the treatments, including that of serum starvation. The electron microscopy images in further confirm that the autophagic vesicle formation that was otherwise induced by either radiation alone or the 1,25D3 + radiation combination, was significantly suppressed in the ATG5 knockdown cells (essentially no autophagic vesicles were detected with any of the indicated treatments).
Figure 4. Silencing of ATG5 in MCF-7 cells. MCF-7 cells were stably transfected with either an empty vector or with a plasmid for the silencing of ATG5. (A). ATG5 levels are shown by western blotting comparing control vector transfected cells to those with ATG5 knockdown; all subsequent studies utilized the shATG5 1-3-3 clones. (B) Vector MCF-7 cells were exposed to radiation alone (5×2 Gy), or 1,25D3 prior to irradiation and autophagy was monitored by acridine orange staining. (C) MCF-7/ATG5- cells were treated identically to vector control cells in B; average number of AVOs per cell were counted in three fields for each condition. (D) LC3-I and LC3-II levels were monitored by western blotting 24 h post-irradiation in MCF-7/ATG5− cells and compared with Control MCF-7 parental cells. Actin was utilized as a loading control. Serum starvation was used as a positive control for autophagic flux. (E) Electron microscopy imaging for autophagosomes. Scale bars indicate magnification.
In further studies to address both the putative cytoprotective actions of autophagy with radiation alone and the putative cytotoxic functions of autophagy for the combination treatment, the MCF-7/ATG5− cells were exposed to either radiation alone or 1,25D3 followed by radiation, and viable cell number was monitored for a period of 12 d after the last dose of radiation (, upper panel). It should be noted that the MCF-7 vector control cells responded to these treatments in essentially an identical manner as parental MCF-7 cells (, lower panel, compared with , respectively); moreover there was no evidence for apoptosis as measured by TUNEL/DAPI in the MCF-7 vector control cells (data not shown). These experiments in large part recapitulated the findings presented in in that the prolonged growth arrest and proliferative recovery that occurs with radiation treatment was abrogated in the ATG5 knockdown cells both for treatment with radiation alone and for treatment with 1,25D3 + radiation. (TUNEL and DAPI staining) indicates that cell death in the MCF-7/ATG5− cells exposed to either radiation alone or 1,25D3 + radiation occurred largely through apoptosis, again similar to the data presented in . p62 degradation was promoted with serum starvation or the 1,25D3 + radiation treatment; however, p62 levels were not diminished by radiation alone ( and accompanying bar graph), suggesting a possible distinction between cytotoxic and cytoprotective autophagy in this experimental model
Figure 5. Influence of 1,25D3 on the response to fractionated radiation in MCF-7/ATG5− cells. (A) ATG5− cells (top panel) or MCF-7 vector control cells (bottom panel) were exposed to radiation alone (5×2 Gy), or 1,25D3 prior to irradiation and viable cell number was determined by exclusion of trypan blue at the indicated days following the initiation of radiation exposure. ↑ Indicates when irradiation occurred. **p < 0.0001 from IR on corresponding day, ┼p < 0.0001 comparing corresponding time points in MCF-7/ATG5− cells and vector control cells. (B) Apoptosis was monitored by the TUNEL assay and DAPI staining 3 d post-irradiation. (C) Autophagic flux was based on the decline in p62 levels monitored by western blotting 24 h post-irradation. Actin was utilized as a loading control. Serum starvation was used as a positive control for autophagic flux. The bar graph presents densitometry data for the p62/β actin protein ratio normalized to controls.
Similar studies were performed in MCF-7/ATG7− cells in which ATG7, another critical autophagy-related gene,Citation42,Citation43 was suppressed (Fig. S3A). Figure S3B indicates the MCF7/ATG7− cells had a relatively high basal level of autophagy, again based on AO staining; however, none of the treatments significantly increased the level of AO puncta. Previous studies indicated that the downregulation of the autophagy protein ATG7 by RNA interference in MCF-7 cells does not interfere with LC3-I to LC3-II processing in both ATG7 knockdown and Scr cells, indicating that ATG7 knockdown cells are capable of LC3 processing, which could allow for autophagosome accumulation.Citation44Figure S3C presents a time-course response to treatment in the ATG7 knockdown cells that confirms increased sensitivity to radiation (sustained arrest and lack of proliferative recovery) as well as high sensitivity to the combination treatment of 1,25D3 + radiation (presumably due largely to the sensitivity to radiation alone (comparing ▲ and ×).
Evidence for the cytotoxic actions of autophagy mediating the actions of 1,25D3 + radiation
In an effort to distinguish between the putative cytoprotective and cytotoxic functions of autophagy in this experimental system, we were able to identify a (reduced) concentration of CQ (5 µM) that produced minimal sensitization to radiation when administered in combination with a single 4 Gy dose of radiation (Fig. S4A) but which was able to protect the cells from the combination treatment of 1,25D3 + radiation (Fig. S4B). Figure S4C demonstrates that 1,25D3, radiation (4 Gy) and 1,25D3 + radiation all had similar effects, respectively, as when studies were performed with fractionated radiation. This series of experiments supports the premise that radiation sensitization by 1,25D3 is likely being mediated through the promotion of autophagy.
Sensitization to radiation in MCF7/HER2 cells
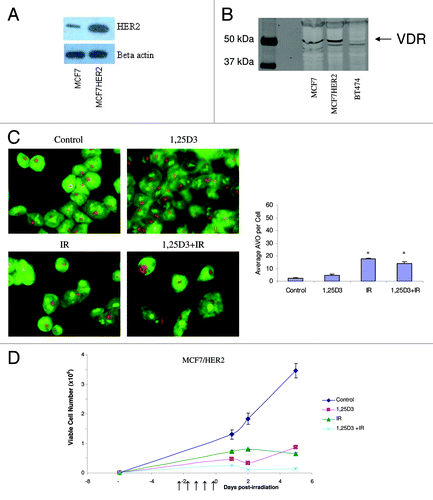
Given that breast cancer cells are generally considered to be relatively sensitive to radiation treatment, we sought to determine if treatment of breast tumor cells that are intrinsically resistant to radiation through the overexpression of HER2/neuCitation22 would also demonstrate sensitization to radiation via the promotion of autophagy. confirms the overexpression of HER2 in this cell line while confirms the expression of the vitamin D receptor. indicates that MCF-7HER2 cells constitutively demonstrated low levels of autophagy staining; a single large vacuole was observed in control and in cells exposed to 1,25D3 while again treatment with either radiation alone or 1,25D3 + radiation resulted in increased staining (bar graph accompanying ). demonstrates that the combination of 1,25D3 + radiation resulted in a more pronounced effect than radiation alone, although this appeared to be due in large part to unexpectedly high sensitivity to the 1,25D3; sensitization was also observed with EB1089 + radiation (data not shown).
Figure 6. Response to 1,25D3 and radiation in MCF-7/HER2 cells. (A). HER2 overexpression is confirmed by western blotting in comparison to MCF-7 parental cells. (B) Vitamin D receptor (VDR) levels were monitored via western blotting in MCF-7 parental cells, MCF-7/HER2 cells and BT474 cells (discussed in ). (C) Cells were exposed to radiation alone (5×2 Gy), or 1,25D3 + irradiation and autophagy was monitored based on acridine orange staining 24 h post-irradiation; for the accompanying bar graph, the average number of AVOs per cell were counted in three fields for each condition. (D). Viable cell number was determined by exclusion of trypan blue at the indicated days following the initiation of radiation exposure. ↑ indicates days on which irradiation was performed.
Sensitization of Hs578t cells
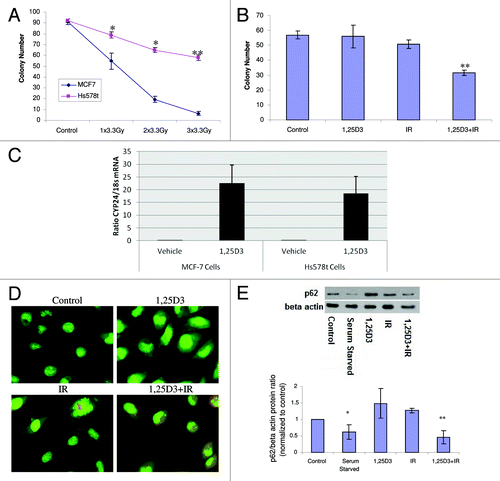
A recent paper by Mineva et al., utilized RT-PCR to confirm the expression level of VDR, and reported radiosensitization by 1,25D3 in Hs578t breast tumor cells,Citation23 a breast tumor cell line that is intrinsically radioresistant.Citation23,Citation24 The clonogenic survival data presented in confirm that Hs578t cells were significantly less radiosensitive than MCF-7 cells. further confirmed the findings of Mineva et al.Citation23 that 1,25D3 enhances radiation sensitivity. Furthermore, Hs578t cells exposed to 1,25D3 alone or radiation alone (5×2 Gy) did not display characteristics of apoptosis, 5 d post-irradiation (Fig. S5). Although apoptosis was significantly increased with 1,25D3 + IR, no more than 15% of these cells were TUNEL positive (Fig. S5 with accompanying bar graph, confirmed with altered nuclear morphology via DAPI), suggesting that autophagy could contribute to 1,25D3 radiosensitization, as in the studies with MCF-7 cells. Since the effect of the combination treatment was relatively modest, induction of CYP24 (24-hydroxylase) gene expression was analyzed to determine the activity of the vitamin D receptor. Hs578t cells expressed functional VDR as demonstrated by a similar induction of CYP24 (24-hydroxylase) gene expression compared with MCF-7 cells (). indicates that the combination of 1,25D3 with radiation resulted in significant autophagy in comparison to control and 1,25D3-treated cells (with autophagy also induced by radiation alone), based on acridine orange staining, findings similar to those with MCF-7 and MCF-7/HER2 cells. Studies of p62 degradation shown in (and the accompanying bar graph) confirm that exposure to 1,25D3 + radiation also promotes significant autophagic flux. It is of further importance to emphasize that radiation alone did not appear to promote p62 degradation in this cell line, which supports the contention that increased autophagic flux is likely to be responsible for radiation sensitization by 1,25D3.
Figure 7. Influence of 1,25D3 on radiation sensitivity and autophagy in Hs578t breast tumor cells. (A) MCF-7 and Hs578t cell were treated with varying doses of radiation and colony formation was assessed. (B) Hs578t cells were exposed to 1,25D3 alone, radiation alone (5×2 Gy), or 1,25D3 prior to irradiation and colony formation was assessed. (C) MCF-7 and Hs578t cells were analyzed in the presence or absence of 1,25D3 for VDR functionality via analysis of inducible CYP24 (24-hydroxylase) gene expression. (D) Autophagy was monitored based on acridine orange staining 24 h post-irradiation. (E) Autophagic flux was based on the decline in p62 levels monitored by western blotting 24 h post-irradiation. Actin was utilized as a loading control. Serum starvation was used as a positive control for autophagic flux. Densitometry for the p62/β actin protein ratio was normalized to controls; *p < 0.05, **p < 0.0001 from control.
Lack of sensitization of BT474 breast tumor cells
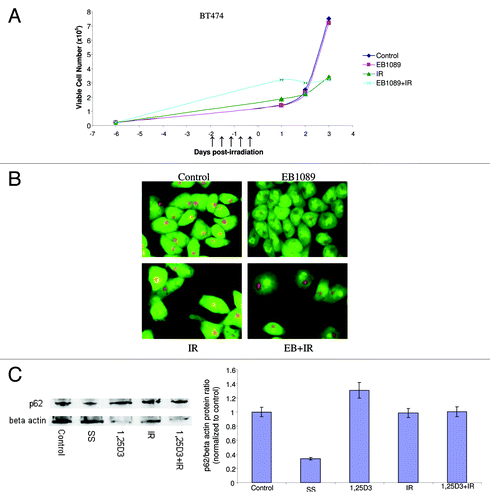
To further examine whether our strategies for radiosensitization would also be effective against intrinsically radioresistant cell lines, we also examined HER2 overexpressing BT474 breast cancer cells.Citation45 indicates the temporal differences in viable cell number between control cells and cells that were treated with either radiation alone or EB1089 followed by radiation. The BT474 cells were clearly quite resistant to radiation treatment, which failed to produce significant effects on cell growth. EB1089 was unable to radiosensitize these cells and upon further examination the combination was unable to elicit an autophagic response as indicated by the lack of change in acridine orange staining in . Studies of p62 degradation shown in (and the accompanying bar graph) further confirm that neither 1,25D3, IR nor 1,25D3 + IR promotes significant autophagic flux. Although the literature indicates that BT474 cells express the VDR and are responsive to vitamin D,Citation25 reveals that BT474 cells actually have minimally detectable levels of the VDR, which supports the necessity for having a relatively robust VDR mediated signaling response pathway for radiosensitization by vitamin D3 or its analogs. The observation that autophagy and autophagic flux are not increased under conditions where 1,25D3 fails to promote radiosensitization lends further support to the contention that radiosensitization by vitamin D is accomplished through modulation of the autophagic pathway.
Figure 8. Lack of response to 1,25D3 and radiation in BT474 breast tumor cells. (A) Cells were exposed to radiation alone (5×2 Gy), or 1,25D3 prior to irradiation and viable cell number was determined by exclusion of trypan blue at the indicated days following the initiation of radiation exposure. ↑ indicates days on which irradiation was performed. (B) Autophagy was monitored based on acridine orange staining 24 h post-irradiation. (C) Autophagic flux was based on the decline in p62 levels monitored by western blotting 24 h post-irradiation. Actin was utilized as a loading control. Serum starvation was used as a positive control for autophagic flux. Densitometry for the p62/β actin protein ratio was normalized to control.
Discussion
The utility of combining with 1,25D3 or vitamin D3 analogs with conventional chemotherapeutic drugs such as tamoxifen, platinum compounds, and Adriamycin against a variety of tumor cell lines is supported by multiple studies.Citation11,Citation12,Citation14-Citation16 In addition, phase I and II clinical trials have suggested that high dose intermittent therapy with 1,25D3 itself is potentially safe.Citation30 Our previous workCitation2-Citation5 has focused on utilizing 1,25D3 and its analogs to confer sensitivity to radiation in the breast tumor cell, apparently through the promotion of autophagy.Citation2,Citation3
The current work is consistent with the premise in a number of studies that radiation promotes cytoprotective autophagy and that radiation sensitization can be mediated through pharmacological or genetic suppression of autophagy. However, our studies are also consistent with the conclusion that autophagy is the basis for enhanced sensitivity and cell death in response to irradiation when irradiation is preceded by either 1,25D3 or the vitamin D analog, EB1089; this conclusion is based on evidence for autophagy via multiple assays that include autophagic vesicle formation, RFP-LC3 redistribution and punctation, electron microscopy and degradation of the p62 protein, as well as lack of evidence for other modes of cell death that was reported in previous studies.Citation2-Citation5 Consequently, in this experimental model, it appears that autophagy may have the capacity to play dual roles, both of which can be exploited to enhance the response to radiation.
An additional complication to confirming the cytotoxic functions of autophagy in this experimental system was that neither pharmacological nor genetic inhibition of autophagy reduced sensitivity to the combination treatment that utilized fractionated radiation (5×2 Gy); this was due to the fact that the irradiated cells die by an alternative pathway, that of apoptosis, which is a frequent observation when autophagy is blocked in tumor cells exposed to stress.Citation18,Citation46,Citation47 However, in ZR-75 breast tumor cells, a cell line where cytoprotective autophagy is relatively weak, the cells are unequivocally protected from the combination treatment by both pharmacologic and genetic autophagy inhibition.Citation48 Using a similar experimental strategy of lowering the concentration of CQ and utilizing a single dose of 4 Gy IR, we were able to dramatically reduce radiation sensitization by CQ, thereby allowing for detection of the capacity of CQ to protect the cells from the cytotoxicity of the 1,25D3 + IR combination treatment. These observations support the conclusion that autophagy mediates the cytotoxicity of the 1,25D3 + IR combination treatment. Additional, albeit somewhat indirect, evidence in support of this premise is provided by the data in that indicate p62 degradation occurs solely for the 1,25D3 + IR combination treatment in Hs578t breast tumor cells.
While there is some evidence that EB1089 or 1,25D3 alone can induce autophagosome formation and autophagic flux,Citation49-Citation53 under the experimental conditions of the current studies, no autophagy is observed with EB1089 or 1,25D3 alone. Our current studies with EB1089 and 1,25D3 demonstrate comparable temporal responses to both agents alone and in combination with irradiation, indicating equivalent utilities for both drugs. We also show that as with EB1089, 1,25D3 suppresses the proliferative recovery that occurs after radiation alone. This component of the response to radiation is potentially of clinical significance since the senescence that otherwise occurs with radiation alone could prove to reflect tumor dormancy that is ultimately succeeded by disease recurrence.Citation34,Citation54
Our studies also indicate that sensitization can occur even in cells that are intrinsically resistant to radiation through overexpression of Her-2/neu. Unexpectedly, MCF7HER2 cells were extremely sensitive to the antiproliferative effects of the 1,25D3. Conversely, an apparent deficiency of the vitamin D receptor in the BT474 cells is likely to be the explanation for their lack of radiosensitization with vitamin D, and the absence of autophagy. Interestingly, Costa et al. have suggested that the vitamin D receptor may be unnecessary for its antiproliferative actions, which could indicate that effects on tumor cell growth and radiation sensitivity are dissociable.Citation55 With regard to the Hs578t cells, we have confirmed the report of radiation resistance and sensitization by 1,25D3; however, similar to the findings by Mineva et al.,Citation23 the extent of sensitization was somewhat lower than for MCF-7 cells. This may be related to the fact that these cells have mutant p53, as we have reported previously that MCF-7/E6 cells (that are essentially null for p53) are relatively refractory to sensitization;Citation5
In summary, these studies are consistent with the possibility that both cytoprotective and cytotoxic autophagy can occur in breast tumor cells exposed to radiation and furthermore that both forms of autophagy could potentially be exploited for the purpose of radiosensitization. Furthermore, the availability of an experimental system where the response to radiation alone is cytoprotective autophagy and the response to the 1,25D3 + IR combination is cytoprotective autophagy could, in theory, provide a platform for identifying the factors that distinguish between these two functions of autophagy.
Materials and Methods
Cell lines
The p53 wild-type (WT) MCF-7 human breast tumor cell line was obtained from National Cancer Institute. BT474 and Hs578t cells were obtained from ATCC. Development of the MCF-7/ATG5−/− cells is described below. The MCF-7 RFP/LC3 cells were a generous gift from Dr. Keith Miskimins.Citation42 The MCF-7/ATG7−/− cells were a generous gift from Dr. Ameeta Kelekar.Citation44
Cell culture and treatment
The MCF-7 cell line was obtained from the National Cancer Institute (http://dtp.nci.nih.gov/branches/btb/tumor-catalog.pdf). All MCF-7 derived cell lines were grown from frozen stocks in basal RPMI 1640 supplemented with 5% FCS, 5% BCS, 2 mmol/L l-glutamine and penicillin/streptomycin (0.5 mL/100 mL medium). MCF-7/ATG5−/− were maintained using (200 µg/ml) puromycin (Sigma, p8833) for resistance. MCF-7/HER2 cells were maintained using (200 µg/ml) G418 (Gibco, 10131-035). BT474 cells were grown from frozen stocks in DMEM supplemented with 10% FCS, 2 mmol/L l-glutamine and penicillin/streptomycin (0.5 mL/100 mL medium). Hs578t cells were grown from frozen stocks in α-DMEM supplemented with 10% FCS, 2 mmol/L l-glutamine and penicillin/streptomycin (0.5 mL/100 mL medium). All cells were maintained at 37°C under a humidified, 5% CO2 atmosphere. Cells were exposed to γ-IR using a 137Cs irradiator. Radiation treatment, unless otherwise stated, consisted of five fractions of 2 Gy radiation that were administered on three consecutive days (two fractions separated by 6 h on the first 2 d followed by a fifth dose on the 3rd day). In our studies, cells were exposed to 100 nmol/L EB1089 (Leo Pharmaceuticals batch no. EB1 262160) or 1,25 vitamin D3 (Sigma, D1530) for 72 h before irradiation. This sequence of exposure was based on the studies by Wang et al.Citation11 and our own previous work,Citation2,Citation4,Citation5 which have shown a requirement for prolonged incubation with vitamin D3 or its analogs to promote sensitivity to Adriamycin and irradiation.
Cell viability and clonogenic survival
Cell viability was determined by trypan blue exclusion at various time points after the last dose of radiation. Cells were harvested using trypsin, stained with 0.4% trypan blue dye (Sigma, T8154), and counted using phase contrast microscopy; a minimum of three experimental replicates were conducted. For clonogenic survival studies, cells were plated in triplicate in six well tissue culture dishes at the appropriate density for each condition. After 14 d, the cells were fixed with 100% methanol, air-dried and stained with 0.1% crystal violet (Sigma, C3886). For computing the survival fraction, groups of 50 or more cells were counted as colonies. Data were normalized relative to untreated controls, which were taken as 100% survival; a minimum of three experimental replicates were conducted. Colonies were imaged and average colony width was measured using image analysis software, QCapturePro.
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling assay for apoptosis
The method of Gavrieli et al.Citation56 was used as an independent assessment of apoptotic cell death in combined cytospins containing both adherent and nonadherent cells. Cells were fixed and the fragmented DNA in cells undergoing apoptosis was detected using the In situ Cell Death Detection kit (Roche, 11373242910, 03333566001), where strand breaks are end-labeled with fluorescein-dUTP by the enzyme terminal transferase. Cells were then fixed to glass slides using DAPI-containing Vectashield mounting medium (Sigma, D9542). Pictures were taken using an Olympus inverted fluorescence microscope. All images presented are at the same magnification; a minimum of five fields of view were counted per condition and a minimum of three experimental replicates were conducted.
Western blot analysis
After the indicated treatments, cells were washed in PBS and lysed using 500–1,000 μL M-PER mammalian protein extraction reagent (Thermo Scientific, 78501) containing protease and phosphatase inhibitors for 5 min on a shaker. Protein concentrations were determined by the Lowry method and equal aliquots of protein (40 μg) were separated using 8–15% SDS-PAGE. Proteins were transferred onto a nitrocellulose membrane and blocked in TBS-Tween 20 buffer containing 5% nonfat dry milk. Membranes were immunoblotted with respective antibodies and then incubated with horseradish peroxidase–conjugated secondary antibody. Proteins were visualized using an enhanced chemiluminescence kit from Pierce (Thermo Scientific, 34080). Primary antibodies used were anti-p62 (SQSTM1, Santa Cruz, sc-28359), anti-ATG5 (APG5, Biosensis, R-111-100), anti-ATG7 (APG7, Santa Cruz, sc-33211), anti-VDR (Santa Cruz, sc-13133), anti-β actin (Santa Cruz, sc-47778), all primary antibodies presented were used at a 1:1,000 dilution. All westerns were replicated a minimum of three times and densitometry comparing proteins of interest to β actin for loading control was conducted via ImageJ.
Detection of autophagic cells by staining with acridine orange
As a marker of autophagy, the volume of the cellular acidic compartment was visualized by acridine orange staining.Citation26 Cells were seeded in six-well tissue culture dishes and treated as described above for the cell viability study. Twenty-four hours following treatment, cells were incubated with medium containing 0.1 μg/mL acridine orange (Invitrogen, A3568) for 15 min; the acridine orange was then removed, cells were washed once with PBS, fresh media was added, and fluorescent micrographs were taken using an Olympus inverted fluorescence microscope. Again, all images presented are at the same magnification. The number of cells with increased acidic vesicular organelles was determined by counting at least three representative fields per treatment condition; a minimum of three replicate experiments were conducted.
Transmission electron microscopy
TEM services, including sample fixation, embedding, ultra-microtomy and staining were provided by the VCU Department of Anatomy and Neurobiology Microscopy Facility. Sections were imaged via Jeol JEM-1230 transmission electron microscope (EM) equipped with a Gatan UltraScan 4000SP 4 K × 4 K CCD camera. The magnification of image is indicated at the bottom of micrograph images; a minimum of 20 representative cells from at least three grids were evaluated for the appearance of autophagosomes.
RNA interference
ATG5 shRNA oligonucleotides were designed according to the sequence described previously.Citation57 For each set, top- and bottom-strand oligos were synthesized separately and annealed together. The primers for siRNA corresponding to the coding region were as follows: top, 5′-gatccccGGATGAGATAACTGAAAGGttcaagagaCCTTTCAGTTATCTCATCCttttta-3′ and bottom, 5′-gatccccGGCATTATCCAATTGGTTTttcaagagaAAACCAATTGGATAATGCCttttta-3′;
The BglII restriction site was included on the top strand at its 5′ end; the HindIII restriction site was included at the 3′ end to facilitate cloning into the pSUPER-retro-puro (OligoEngine, VEC-PRT-0002). The 2 oligos were annealed and inserted into the empty vector that has been linearized with BglII and HindIII enzymes. Positive clones were identified and sequenced and confirmed by automated DNA sequencing.
Gene expression analysis
For Q-PCR, total RNA was collected from washed cultured cells lysed in TRIzol (Invitrogen). Treatment with DNase I was used to remove contaminating DNA before the PCR was performed. One microgram of total RNA was batch reverse transcribed using Supercript II and PCR amplified with the specified human primer sets for human CYP24 (Forward, Sequence: TATCGCGACTACCGCAAAGA; Reverse, Sequence: GGACCCGCTGCCAGTCT), and h18s rRNA (supplied with MASTER MIX 20X, from Applied Biosystems, part number 4310884E) were synthesized at the VCU Nucleic Acids Core Facility. The Q-PCR experiments were performed in the ABI Prism® 7900 Sequence Detection System (Applied Biosystems) using the TaqMan® One Step PCR Master Mix Reagents Kit. All the samples were tested in triplicate. The cycling conditions were: 48°C/30 min; 95°C/10 min; and 40 cycles of 95°C/15 sec and 60°C/1 min. The cycle threshold was determined to provide the optimal standard curve values (0.98 to 1.0). The probes and primers were designed using the Primer Express® 3.0 version. The probes were labeled in the 5′ end with FAM (6-carboxyfluoresceine) and in the 3′ end with TAMRA (6-carboxytetramethylrhodamine). Specific gene expression analysis was conducted using TaqMan probes to gene products of interest, CYP24 (CCTTCCAGGATCAGCAGCCCGTAG) and 18s rRNA.
Statistical analysis
Statistical differences were determined using StatView statistical software. Comparisons were made using a one-way ANOVA followed by Fisher’s protected least significance difference post-hoc test. ps ≤ 0.05 were taken as statistically significant.
| Abbreviations: | ||
| 1 | = | 25D3,1,25 dihydroxyvitamin D3 |
| DAPI | = | 4′,6-diamidino-2-phenylindole |
| AO | = | acridine orange |
| AVOs | = | autophagic vacuoles |
| CQ | = | chloroquine |
| IR | = | radiation |
| TUNEL | = | terminal deoxynucleotidyl transferase dUTP nick end labeling |
| EM | = | transmission electron microscopy |
| VDR | = | vitamin D receptor |
Additional material
Download Zip (386.8 KB)Acknowledgments
This work was supported, in part, by American Institute for Cancer Research Grant #06A058-REV. Molly Bristol was supported, in part, by a predoctoral training grant (W81XWH-09-1-0020) from the Department of Defense. Electron microscopy was performed at the VCU—Department of Neurobiology and Anatomy Microscopy Facility, supported, in part, with funding from NIH-NINDS Center core grant P30NS047463. The RFP-LC3 vector was generously provided by Dr. Keith Miskimins at the University of South Dakota and was originally developed by the laboratory of Dr. A.M. Tolkovsky. The MCF7/ATG7−/− cells were a generous gift from the lab of Dr. Ameeta Kelekar at University of Minnesota, Minneapolis, MN.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Notes
* Although this representative experiment appears to indicate differences in the extent of p62 degradation between IR alone and 1,25D3 + IR, densitometry analysis and pooling of the data from multiple western blots has determined that the level of p62 degradation is not significantly different between these treatments.
References
- Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol 2010; 28:3271 - 7; http://dx.doi.org/10.1200/JCO.2009.25.9820; PMID: 20498394
- DeMasters GA, Gupta MS, Jones KR, Cabot M, Wang H, Gennings C, et al. Potentiation of cell killing by fractionated radiation and suppression of proliferative recovery in MCF-7 breast tumor cells by the Vitamin D3 analog EB 1089. J Steroid Biochem Mol Biol 2004; 92:365 - 74; http://dx.doi.org/10.1016/j.jsbmb.2004.07.011; PMID: 15698541
- Demasters G, Di X, Newsham I, Shiu R, Gewirtz DA. Potentiation of radiation sensitivity in breast tumor cells by the vitamin D3 analogue, EB 1089, through promotion of autophagy and interference with proliferative recovery. Mol Cancer Ther 2006; 5:2786 - 97; http://dx.doi.org/10.1158/1535-7163.MCT-06-0316; PMID: 17121925
- Chaudhry M, Sundaram S, Gennings C, Carter H, Gewirtz DA. The vitamin D3 analog, ILX-23-7553, enhances the response to adriamycin and irradiation in MCF-7 breast tumor cells. Cancer Chemother Pharmacol 2001; 47:429 - 36; http://dx.doi.org/10.1007/s002800000251; PMID: 11391859
- Sundaram S, Sea A, Feldman S, Strawbridge R, Hoopes PJ, Demidenko E, et al. The combination of a potent vitamin D3 analog, EB 1089, with ionizing radiation reduces tumor growth and induces apoptosis of MCF-7 breast tumor xenografts in nude mice. Clin Cancer Res 2003; 9:2350 - 6; PMID: 12796405
- Hansen CM, Hamberg KJ, Binderup E, Binderup L. Seocalcitol (EB 1089): a vitamin D analogue of anti-cancer potential. Background, design, synthesis, pre-clinical and clinical evaluation. Curr Pharm Des 2000; 6:803 - 28; http://dx.doi.org/10.2174/1381612003400371; PMID: 10828309
- Matthews D, LaPorta E, Zinser GM, Narvaez CJ, Welsh J. Genomic vitamin D signaling in breast cancer: Insights from animal models and human cells. J Steroid Biochem Mol Biol 2010; 121:362 - 7; http://dx.doi.org/10.1016/j.jsbmb.2010.03.061; PMID: 20412854
- Gocek E, Studzinski GP. Vitamin D and differentiation in cancer. Crit Rev Clin Lab Sci 2009; 46:190 - 209; http://dx.doi.org/10.1080/10408360902982128; PMID: 19650715
- Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr 2008; 88:582S - 6S; PMID: 18689406
- Schwartz GG, Eads D, Naczki C, Northrup S, Chen T, Koumenis C. 19-nor-1 alpha,25-dihydroxyvitamin D2 (paricalcitol) inhibits the proliferation of human pancreatic cancer cells in vitro and in vivo. Cancer Biol Ther 2008; 7:430 - 6; http://dx.doi.org/10.4161/cbt.7.3.5418; PMID: 18094617
- Wang Q, Yang W, Uytingco MS, Christakos S, Wieder R. 1,25-Dihydroxyvitamin D3 and all-trans-retinoic acid sensitize breast cancer cells to chemotherapy-induced cell death. Cancer Res 2000; 60:2040 - 8; PMID: 10766196
- Vink-van Wijngaarden T, Pols HA, Buurman CJ, van den Bemd GJ, Dorssers LC, Birkenhäger JC, et al. Inhibition of breast cancer cell growth by combined treatment with vitamin D3 analogues and tamoxifen. Cancer Res 1994; 54:5711 - 7; PMID: 7923220
- Thompson T, Andreeff M, Studzinski GP, Vassilev LT. 1,25-dihydroxyvitamin D3 enhances the apoptotic activity of MDM2 antagonist nutlin-3a in acute myeloid leukemia cells expressing wild-type p53. Mol Cancer Ther 2010; 9:1158 - 68; http://dx.doi.org/10.1158/1535-7163.MCT-09-1036; PMID: 20406950
- Light BW, Yu WD, McElwain MC, Russell DM, Trump DL, Johnson CS. Potentiation of cisplatin antitumor activity using a vitamin D analogue in a murine squamous cell carcinoma model system. Cancer Res 1997; 57:3759 - 64; PMID: 9288784
- Ravid A, Rocker D, Machlenkin A, Rotem C, Hochman A, Kessler-Icekson G, et al. 1,25-Dihydroxyvitamin D3 enhances the susceptibility of breast cancer cells to doxorubicin-induced oxidative damage. Cancer Res 1999; 59:862 - 7; PMID: 10029076
- Hershberger PA, Yu WD, Modzelewski RA, Rueger RM, Johnson CS, Trump DL. Calcitriol (1,25-dihydroxycholecalciferol) enhances paclitaxel antitumor activity in vitro and in vivo and accelerates paclitaxel-induced apoptosis. Clin Cancer Res 2001; 7:1043 - 51; PMID: 11309356
- Jones KR, Elmore LW, Jackson-Cook C, Demasters G, Povirk LF, Holt SE, et al. p53-Dependent accelerated senescence induced by ionizing radiation in breast tumour cells. Int J Radiat Biol 2005; 81:445 - 58; http://dx.doi.org/10.1080/09553000500168549; PMID: 16308915
- Boya P, González-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, et al. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol 2005; 25:1025 - 40; http://dx.doi.org/10.1128/MCB.25.3.1025-1040.2005; PMID: 15657430
- Livesey KM, Tang D, Zeh HJ, Lotze MT. Autophagy inhibition in combination cancer treatment. Curr Opin Investig Drugs 2009; 10:1269 - 79; PMID: 19943199
- Lambert LA, Qiao N, Hunt KK, Lambert DH, Mills GB, Meijer L, et al. Autophagy: a novel mechanism of synergistic cytotoxicity between doxorubicin and roscovitine in a sarcoma model. Cancer Res 2008; 68:7966 - 74; http://dx.doi.org/10.1158/0008-5472.CAN-08-1333; PMID: 18829554
- Wu YT, Tan HL, Huang Q, Kim YS, Pan N, Ong WY, et al. Autophagy plays a protective role during zVAD-induced necrotic cell death. Autophagy 2008; 4:457 - 66; PMID: 18253089
- Liang K, Lu Y, Jin W, Ang KK, Milas L, Fan Z. Sensitization of breast cancer cells to radiation by trastuzumab. Mol Cancer Ther 2003; 2:1113 - 20; PMID: 14617784
- Mineva ND, Wang X, Yang S, Ying H, Xiao ZX, Holick MF, et al. Inhibition of RelB by 1,25-dihydroxyvitamin D3 promotes sensitivity of breast cancer cells to radiation. J Cell Physiol 2009; 220:593 - 9; http://dx.doi.org/10.1002/jcp.21765; PMID: 19373868
- Tian K, Wang Y, Xu H. WTH3 is a direct target of the p53 protein. Br J Cancer 2007; 96:1579 - 86; http://dx.doi.org/10.1038/sj.bjc.6603724; PMID: 17426708
- Mehta RG, Hussain EA, Mehta RR, Das Gupta TK. Chemoprevention of mammary carcinogenesis by 1alpha-hydroxyvitamin D5, a synthetic analog of Vitamin D. Mutat Res 2003; 523-524:253 - 64; http://dx.doi.org/10.1016/S0027-5107(02)00341-X; PMID: 12628523
- Paglin S, Hollister T, Delohery T, Hackett N, McMahill M, Sphicas E, et al. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res 2001; 61:439 - 44; PMID: 11212227
- Kirisako T, Ichimura Y, Okada H, Kabeya Y, Mizushima N, Yoshimori T, et al. The reversible modification regulates the membrane-binding state of Apg8/Aut7 essential for autophagy and the cytoplasm to vacuole targeting pathway. J Cell Biol 2000; 151:263 - 76; http://dx.doi.org/10.1083/jcb.151.2.263; PMID: 11038174
- Bursch W, Ellinger A, Gerner C, Fröhwein U, Schulte-Hermann R. Programmed cell death (PCD). Apoptosis, autophagic PCD, or others?. Ann N Y Acad Sci 2000; 926:1 - 12; http://dx.doi.org/10.1111/j.1749-6632.2000.tb05594.x; PMID: 11193023
- Hansen CM, Binderup L, Hamberg KJ, Carlberg C. Vitamin D and cancer: effects of 1,25(OH)2D3 and its analogs on growth control and tumorigenesis. Front Biosci 2001; 6:D820 - 48; http://dx.doi.org/10.2741/Hansen; PMID: 11438443
- Trump DL, Potter DM, Muindi J, Brufsky A, Johnson CS. Phase II trial of high-dose, intermittent calcitriol (1,25 dihydroxyvitamin D3) and dexamethasone in androgen-independent prostate cancer. Cancer 2006; 106:2136 - 42; http://dx.doi.org/10.1002/cncr.21890; PMID: 16598750
- Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem 2007; 282:24131 - 45; http://dx.doi.org/10.1074/jbc.M702824200; PMID: 17580304
- Hartley T, Brumell J, Volchuk A. Emerging roles for the ubiquitin-proteasome system and autophagy in pancreatic beta-cells. Am J Physiol Endocrinol Metab 2009; 296:E1 - 10; http://dx.doi.org/10.1152/ajpendo.90538.2008; PMID: 18812463
- Nedelsky NB, Todd PK, Taylor JP. Autophagy and the ubiquitin-proteasome system: Collaborators in neuroprotection. Biochim Biophys Acta 2008; 1782:691-699.
- Gewirtz DA, Hilliker ML, Wilson EN. Promotion of autophagy as a mechanism for radiation sensitization of breast tumor cells. Radiother Oncol 2009; 92:323 - 8; http://dx.doi.org/10.1016/j.radonc.2009.05.022; PMID: 19541381
- Bursch W, Ellinger A, Kienzl H, Török L, Pandey S, Sikorska M, et al. Active cell death induced by the anti-estrogens tamoxifen and ICI 164 384 in human mammary carcinoma cells (MCF-7) in culture: the role of autophagy. Carcinogenesis 1996; 17:1595 - 607; http://dx.doi.org/10.1093/carcin/17.8.1595; PMID: 8761415
- Solomon VR, Lee H. Chloroquine and its analogs: a new promise of an old drug for effective and safe cancer therapies. Eur J Pharmacol 2009; 625:220 - 33; http://dx.doi.org/10.1016/j.ejphar.2009.06.063; PMID: 19836374
- Shacka JJ, Klocke BJ, Roth KA. Autophagy, bafilomycin and cell death: the “a-B-cs” of plecomacrolide-induced neuroprotection. Autophagy 2006; 2:228 - 30; PMID: 16874105
- Zhao H, Cai Y, Santi S, Lafrenie R, Lee H. Chloroquine-mediated radiosensitization is due to the destabilization of the lysosomal membrane and subsequent induction of cell death by necrosis. Radiat Res 2005; 164:250 - 7; http://dx.doi.org/10.1667/RR3436.1; PMID: 16137197
- Apel A, Herr I, Schwarz H, Rodemann HP, Mayer A. Blocked autophagy sensitizes resistant carcinoma cells to radiation therapy. Cancer Res 2008; 68:1485 - 94; http://dx.doi.org/10.1158/0008-5472.CAN-07-0562; PMID: 18316613
- Lomonaco SL, Finniss S, Xiang C, Decarvalho A, Umansky F, Kalkanis SN, et al. The induction of autophagy by gamma-radiation contributes to the radioresistance of glioma stem cells. Int J Cancer 2009; 125:717 - 22; http://dx.doi.org/10.1002/ijc.24402; PMID: 19431142
- Zois CE, Koukourakis MI. Radiation-induced autophagy in normal and cancer cells: towards novel cytoprotection and radio-sensitization policies?. Autophagy 2009; 5:442 - 50; http://dx.doi.org/10.4161/auto.5.4.7667; PMID: 19164950
- Bampton ET, Goemans CG, Niranjan D, Mizushima N, Tolkovsky AM. The dynamics of autophagy visualized in live cells: from autophagosome formation to fusion with endo/lysosomes. Autophagy 2005; 1:23 - 36; http://dx.doi.org/10.4161/auto.1.1.1495; PMID: 16874023
- Mizushima N, Noda T, Yoshimori T, Tanaka Y, Ishii T, George MD, et al. A protein conjugation system essential for autophagy. Nature 1998; 395:395 - 8; http://dx.doi.org/10.1038/26506; PMID: 9759731
- Separovic D, Kelekar A, Nayak AK, Tarca AL, Hanada K, Pierce JS, et al. Increased ceramide accumulation correlates with downregulation of the autophagy protein ATG-7 in MCF-7 cells sensitized to photodamage. Arch Biochem Biophys 2010; 494:101 - 5; http://dx.doi.org/10.1016/j.abb.2009.11.023; PMID: 19944062
- Monazzam A, Razifar P, Ide S, Rugaard Jensen M, Josephsson R, Blomqvist C, et al. Evaluation of the Hsp90 inhibitor NVP-AUY922 in multicellular tumour spheroids with respect to effects on growth and PET tracer uptake. Nucl Med Biol 2009; 36:335 - 42; http://dx.doi.org/10.1016/j.nucmedbio.2008.12.009; PMID: 19324279
- Amaravadi RK, Yu D, Lum JJ, Bui T, Christophorou MA, Evan GI, et al. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J Clin Invest 2007; 117:326 - 36; http://dx.doi.org/10.1172/JCI28833; PMID: 17235397
- González-Polo RA, Niso-Santano M, Ortíz-Ortíz MA, Gómez-Martín A, Morán JM, García-Rubio L, et al. Inhibition of paraquat-induced autophagy accelerates the apoptotic cell death in neuroblastoma SH-SY5Y cells. Toxicol Sci 2007; 97:448 - 58; http://dx.doi.org/10.1093/toxsci/kfm040; PMID: 17341480
- Wilson EN, Bristol ML, Di X, Maltese WA, Koterba K, Beckman MJ, et al. A switch between cytoprotective and cytotoxic autophagy in the radiosensitization of breast tumor cells by chloroquine and vitamin D. Horm Cancer 2011; 2:272 - 85; http://dx.doi.org/10.1007/s12672-011-0081-7; PMID: 21887591
- Høyer-Hansen M, Bastholm L, Mathiasen IS, Elling F, Jäättelä M. Vitamin D analog EB1089 triggers dramatic lysosomal changes and Beclin 1-mediated autophagic cell death. Cell Death Differ 2005; 12:1297 - 309; http://dx.doi.org/10.1038/sj.cdd.4401651; PMID: 15905882
- Høyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, et al. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell 2007; 25:193 - 205; http://dx.doi.org/10.1016/j.molcel.2006.12.009; PMID: 17244528
- Tavera-Mendoza L, Wang TT, Lallemant B, Zhang R, Nagai Y, Bourdeau V, et al. Convergence of vitamin D and retinoic acid signalling at a common hormone response element. EMBO Rep 2006; 7:180 - 5; http://dx.doi.org/10.1038/sj.embor.7400594; PMID: 16322758
- Wang J, Lian H, Zhao Y, Kauss MA, Spindel S. Vitamin D3 induces autophagy of human myeloid leukemia cells. J Biol Chem 2008; 283:25596 - 605; http://dx.doi.org/10.1074/jbc.M801716200; PMID: 18628207
- Yuk JM, Shin DM, Lee HM, Yang CS, Jin HS, Kim KK, et al. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe 2009; 6:231 - 43; http://dx.doi.org/10.1016/j.chom.2009.08.004; PMID: 19748465
- Beauséjour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P, et al. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J 2003; 22:4212 - 22; http://dx.doi.org/10.1093/emboj/cdg417; PMID: 12912919
- Costa JL, Eijk PP, van de Wiel MA, ten Berge D, Schmitt F, Narvaez CJ, et al. Anti-proliferative action of vitamin D in MCF7 is still active after siRNA-VDR knock-down. BMC Genomics 2009; 10:499; http://dx.doi.org/10.1186/1471-2164-10-499; PMID: 19863778
- Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 1992; 119:493 - 501; http://dx.doi.org/10.1083/jcb.119.3.493; PMID: 1400587
- Fung C, Lock R, Gao S, Salas E, Debnath J. Induction of autophagy during extracellular matrix detachment promotes cell survival. Mol Biol Cell 2008; 19:797 - 806; http://dx.doi.org/10.1091/mbc.E07-10-1092; PMID: 18094039