Abstract
CISD2, an ER BCL2-associated autophagy regulator also known as NAF-1, is responsible for the human degenerative disorder Wolfram Syndrome 2. In order to interrogate the physiological role of CISD2 we generated and characterized the Cisd2 gene deletion in mice. Cisd2 null mice manifest significant degeneration in skeletal muscle tissues, which is accompanied with augmented autophagy, dysregulated Ca2+ homeostasis and elongated mitochondria. Our findings describe a novel role for BCL2-CISD2 in the homeostatic maintenance of skeletal muscle. It remains to be elucidated how and if the antagonism of the BECN1 autophagy-initiating complex and modulation of ER Ca2+ homeostasis by BCL2-CISD2 are interconnected.
In a previous screen to identify endoplasmic reticulum (ER) proteins that constitutively interact with BCL2 located at this membrane, we detected a resident ER protein that was required for BCL2 at the ER to antagonize macroautophagy (herein referred to as autophagy) but, remarkably, was not required for BCL2 to overcome the ability of a resident ER BH3-only protein, BIK, to induce the activation of caspases when overexpressed. In the transformed epithelial cells under study, this new BCL2-BCL2L1-associated protein, which we named nutrient-deprivation autophagy factor-1 (official name CDGSH iron sulfur domain 2, CISD2), allows BCL2 to antagonize autophagy in response to nutrient stress. During starvation, BCL2 acts to inhibit autophagy via direct association with the autophagy effector protein, BECN1. In H1299 lung carcinoma cells, CISD2 contributes to the physical interaction between BECN1 and BCL2, thereby hindering formation of the BECN1-PIK3C3 autophagosome-initiating complex in response to nutrient stress. In addition, the BCL2-CISD2 complex associates with the ER inositol 1,4,5-triphosphate receptor (ITPR/IP3R) and is required for BCL2-mediated depression of ER calcium (Ca2+) stores. Although the relationship between the regulation of ER Ca2+ homeostasis and autophagic responses have been widely studied, very little mechanistic details have yet to emerge.
In humans, a point mutation in the gene encoding CISD2 is the underlying cause of Wolfram Syndrome 2, a rare degenerative disorder with extensive clinical manifestations. To investigate the functional contribution of CISD2 within a physiological context, we generated Cisd2 gene-deleted mice. While Cisd2 null mice display evident clinical signs of degeneration at 2–3 mo of age, we detected the earliest manifestation of degeneration in skeletal muscle, particularly in diaphragm tissue. We observed significant disintegration of the sarcomeric structure and the presence of numerous vacuolar structures in diaphragm muscles of Cisd2 null mice. Accordingly, muscle performance of Cisd2−/− mice is impaired and muscles exhibit a shift toward type I slow-twitch fibers (a feature also noted by other investigators in Bcl2−/− mice). Muscular degeneration is accompanied by enhanced basal autophagy in Cisd2−/− skeletal muscle tissues and tissue-derived primary myoblasts. In addition, consistent with our previous observations, loss of CISD2 results in dysregulated intracellular Ca2+ homeostasis resulting in higher ER and cytoplasmic Ca2+ content in Cisd2 knockout primary myoblasts and myotubes compared with wild type.
Whereas we previously demonstrated a requirement for the BCL2-CISD2 complex in the regulation of autophagy stimulated by nutrient deprivation in cultured cells, this study reveals a role for BCL2-CISD2 in maintaining homeostatic basal levels of autophagy in skeletal muscle. Notably, we also reported the presence of enlarged and elongated mitochondria with extensive compact cristae structures in Cisd2 null muscle tissues and myoblasts. Such changes in mitochondrial morphology have previously been described by Scorrano and colleagues and were attributed to an adaptive response during prolonged nutrient deprivation conditions to maintain mitochondrial integrity, sustain ATP production and cell viability, and potentially to represent a morphology that resists mitophagy. This observation is intriguing as, in our previous study, using short-term (4 h) starvation conditions as a stimulus for autophagy, we did not detect any differences in mitochondrial morphology between Cisd2 knockdown and control epithelial cells. The adaptive response by mitochondria may be linked either to prolonged stress conditions or other cellular contexts. In Cisd2 null muscle, deletion of Cisd2 likely results in accumulated stress over the life span of the animals, thus mimicking stress-induced autophagy; such irremediable stress might also extend to the primary myoblasts derived from Cisd2−/− skeletal muscle. Given that mitochondria and ER are structurally connected to allow intercommunication via Ca2+ signals, an important question for future consideration will be the role of dysregulated Ca2+ homeostasis on the signaling pathway(s) that result in mitochondrial elongation.
While BCL2 regulation of Ca2+ signaling in apoptotic cell death programs has been well explored, the role of BCL2-mediated Ca2+ signals in autophagy remains unclear. As well, it has yet to be determined if and how the regulation of ER Ca2+ homeostasis by the BCL2-CISD2 complex is linked to its role in the antagonism of BECN1. We predict that these two pathways are operating in concert to maintain basal autophagy levels and muscle homeostasis. Identifying potential targets of Ca2+ signaling that modulate the activity of the BECN1 phagophore complex and steps beyond will be key to advancing our understanding of the interplay between Ca2+ and autophagy regulated by BCL2-CISD2.
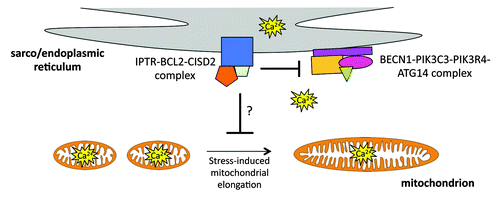
CISD2, like BCL2, associates with the ITPR Ca2+ leak channel at the ER. Whether or not CISD2 influences ITPR function independently of BCL2-BCL2L1 remains unknown. It has been suggested that the ITPR is involved in the modulation of autophagy by serving as a structural platform at the ER to regulate the interaction between BCL2 and BECN1. Another possibility is that the association between BCL2-CISD2 with the ITPR positions BCL2-CISD2 at the nexus of BCL2’s interaction with BECN1 and ER Ca2+ homeostasis. At this location, BCL2-CISD2 may potentially affect Ca2+ levels at the ER, cytosol and mitochondria with consequences on both autophagy and mitochondrial function (see ).
Figure 1. At the sarco/endoplasmic reticulum (SR/ER), the BCL2-CISD2 complex associates with the ITPR Ca2+ channel. In this setting, BCL2-CISD2 may negatively modulate the BECN1 phagophore-initiating complex and regulate ER Ca2+ homeostasis. It remains to be determined if ensuing changes in Ca2+ levels at the ER, cytosol and mitochondria signal changes in the activity of the BECN1 complex, autophagy status and mitochondrial morphology.
Lastly, it is clearly evident that the Cisd2 gene is essential to mammalian physiology. Our studies have demonstrated that the function of CISD2 is intimately linked to the function of BCL2 at the ER, however, BCL2-independent functions of CISD2 may also exist. It also remains to be determined if deletion of the Cisd2 gene in mice recapitulates Wolfram Syndrome 2 in human patients. Interestingly, the role of CISD2 in BCL2-mediated regulation of ER Ca2+ stores correlates with molecular studies demonstrating that WFS1/wolframin, the protein encoded by the gene responsible for Wolfram Syndrome 1, similarly modulates ER Ca2+ load. Does the phenotype of dysregulated Ca2+ homeostasis, mitochondrial elongation and augmented basal autophagy in Cisd2 null mice extend to Wolfram Syndrome 1? Is there potential for therapeutics that modulate the BCL2-CISD2 pathway for treatment of Wolfram syndromes and muscle pathologies?