Abstract
A hallmark of macroautophagy is the formation of autophagosomes, double-membrane vesicles that enwrap cellular components destined for lysosomal degradation. We examined autophagosomal protein dynamics under various inducing stimuli using a comprehensive mass spectrometry-based proteomics approach in combination with functional studies in yeast and human cell cultures. Time frame and stimuli type influenced the autophagosome proteome, underlining the dynamic constitution of the organelle. We identified both a core set of proteins always localizing to autophagosomes and stimulus-dependent components that will serve as a resource for further characterization of the autophagosomal machinery and cargo selection. Among the core proteins were newly discovered autophagy regulators found to be conserved from yeast to humans, as well as the proteasome.
Cellular waste management is primarily handled by the proteasome and the autophagosome-lysosome system. Autophagosomes are double-membrane vesicles that collect and transport components destined for lysosomal degradation. They can be thought of as cellular garbage trucks that collect items for recycling, but the process of selecting items and the molecular composition of the autophagosomal machinery remains elusive. The identification of organelle-specific autophagy subtypes, such as reticulophagy, pexophagy and mitophagy, as well as the identification of autophagy receptors, e.g., SQSTM1/p62, NBR1, BNIP3L/NIX and OPTN/optineurin, and targeting of polyubiquitinated substrates to nascent autophagosomes, also question the concept of nonspecific bulk degradation via macroautophagy. In all cases, newly formed autophagosomes play a vital role. To learn more about autophagosomal composition and substrate targeting we conducted global, unbiased mass spectrometry (MS)-based proteomics experiments. We purified autophagosomes after macroautophagy induction via amino acid starvation and rapamycin treatment, and after macroautophagy blockage using concanamycin A, an inhibitor of the vacuolar H+-ATPase. Autophagosomes were enriched in two ways: (1) by gradient centrifugation and analysis of respective fractions by protein correlation profiling-stable isotope labeling by amino acids in cell culture (PCP-SILAC), and (2) by immunopurification using an anti-eGFP antibody in cells expressing eGFP-LC3. Proteomics analyses identified a core set of proteins that localizes to autophagosomes under all conditions. However, the inducing stimuli as well as the time frame of stimulation are mirrored in different autophagosomal proteome compositions (). Thus, the autophagosome is a highly dynamic organelle and its proteome differs if cells undergo stresses, such as during starvation conditions, or if basal macroautophagy is blocked like with concanamycin A.
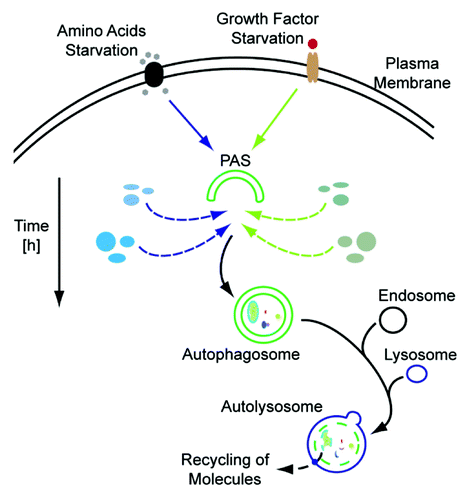
Figure 1. Autophagosomal protein dynamics. Depending on the inducing stimulus, e.g., amino acid deprivation (left side) or growth factor starvation (right side), the autophagosomal protein composition may vary. Degradation of cellular material which is not needed during the respective condition, exemplified by blue and green ellipses for amino acid and growth factor starvation, respectively, will be preferred. In addition, the time frame of stimulation is also reflected by the autophagosomal proteome. PAS, phagophore assembly site.
We focused on the commonly identified autophagosomal proteins. These can in principle be divided into two groups: (a) common autophagosomal cargo destined for lysosmal degradation and (b) autophagy regulators functioning at the autophagosome scaffold. To discriminate the latter from the former we turned to the yeast Saccharomyces cerevisiae and used knockout strains of the respective human homologs to screen for altered autophagy activity after rapamycin treatment or specific nitrogen starvation. We identified five proteins which have so far not been linked to autophagy, four new autophagy activators and one inhibitor. Interestingly, the knockout of vacuolar protein sorting protein Vps35 leads to reduced autophagy activity as determined by the Pho8Δ60-dependent alkaline phosphatase assay. Vps35 is a member of the retromer complex, which is responsible for retrograde transport of cargo from endosomes to the trans-Golgi network and is composed of the selective subunits Vps26, Vps29 and Vps35 and the sorting nexin subunits Vps5 and Vps17. Also, strains missing the components Vps5, Vps17 and Vps29 show reduced autophagy. Hence, it appears that the retromer is actively modulating autophagy in yeast possibly playing a role in membrane trafficking. Moving back to the human system, we could validate the finding in yeast that eukaryotic translation elongation factor 1 gamma (EEF1G) is a positive regulator of autophagy signaling upstream of MTOR.
We also identified an interesting link between the proteasome and autophagy, the proteasome seeming to be one of the “favorite substrates” of autophagosomes—an observation already made by others. We identified proteasomal proteins associated with autophagosomes irrespective of the stimuli used. During active autophagy, proteasomal protein abundance, as well as proteasome activity decreases in cells. However, the molecular mechanisms underlying autophagy-proteasome crosstalk are still not fully uncovered. Thus, it remains unknown if proteasomes are active inside autophagosomes and if autophagosomes can be regarded as scaffolds bringing together the proteasome with its substrates. As we identified basically all components of the 20S proteasome this might be a valid option. Regardless of the mechanism, it becomes clear that there is a balance between the two degradation systems, which can be shifted in favor of one or the other. We also observed this for yeast: rapamycin increases levels of autophagy in temperature sensitive pre1-1 or pre2-1 mutants, which express inactive proteasomes upon a temperature rise, compared with wild-type strains treated with rapamycin. The pre1-1 pre2-1 double-mutant displays synergistic effects.
Taken together, by employing an unbiased organellar proteomics approach we studied the dynamics of autophagosomes in response to different cues. We highlighted stimulus differences and similarities shedding new light on macroautophagy as a targeted degradation process. Combining yeast and human cell culture systems, new conserved autophagy regulators could be identified and the crosstalk between the autophagosome on the one hand and the retromer and the proteasome on the other hand was studied. To our opinion, global ‘omics’ approaches in combination with data modeling hold the promise to further delineate macroautophagy and underlying spatiotemporal protein dynamics. Our study provides a resource of proteins implicated in autophagy either as substrates or components of the autophagosomal machinery—this data set gives a glimpse of the autophagosomal food-chain and will hopefully aid further studies in delineating the signals and mechanisms that govern spatiotemporal specificities of cargo selection during (stress-induced) macroautophagy.