Abstract
Autophagy is an evolutionarily conserved catabolic process that involves the engulfment of cytoplasmic contents in a closed double-membrane structure, called the autophagosome, and their subsequent delivery to the vacuole/lysosomes for degradation. Genetic screens in Saccharomyces cerevisiae have identified more than 30 autophagy-related (Atg) genes that are essential for autophagosome formation. Here we isolated a novel autophagy gene, epg-9, whose loss of function causes defective autophagic degradation of a variety of protein aggregates during C. elegans embryogenesis. Mutations in epg-9 also reduce survival of animals under food depletion conditions. epg-9 mutants exhibit autophagy phenotypes characteristic of those associated with loss of function of unc-51/Atg1 and epg-1/Atg13. epg-9 encodes a protein with significant homology to mammalian ATG101. EPG-9 directly interacts with EPG-1/Atg13. Our study indicates that EPG-9 forms a complex with EPG-1 in the aggrephagy pathway in C. elegans.
Keywords: :
Introduction
Autophagy is a lysosome-mediated degradation process for recycling cytoplasmic contents in response to cellular stresses such as starvation.Citation1,Citation2 It also functions as a quality control system in selectively removing aggregate-prone proteins, damaged organelles and invading pathogens. Genetic screens in yeast have identified more than 30 ATG genes that are required for starvation-induced autophagy and also for the Cvt pathway, an autophagy-related process involved in the transport of vacuolar proteases including aminopeptidase I and α-mannosidase, under vegetative conditions.Citation1,Citation2 Atg proteins have been classified into several groups that act at discrete steps of autophagosome formation. These groups include the Atg1 serine/threonine kinase complex, the Vps34 phosphatidylinositol 3-kinase complex, the Atg8 (ubiquitin-like molecule)/phosphatidylethanolamine (PE) conjugation system, the Atg12 (ubiquitin-like molecule)/Atg5 conjugation system and the Atg9 cycling complex.Citation1,Citation2 In yeast, all Atg proteins associate at least transiently with the phagophore assembly site (PAS), the site at which autophagosomes are thought to be generated.Citation3 In multicellular organisms, the autophagy pathway involves more complex membrane dynamics.Citation4 Most Atg proteins are conserved from yeast to mammals, while the function of some autophagy proteins, including Atg13 and Atg14, are mediated by highly divergent homologs in metazoans.Citation4 The more elaborate autophagy machinery in multicellular organisms also requires essential autophagy genes that are absent in yeast.Citation5
The Atg1/ULK1 (the mammalian Atg1 homolog) complex acts at multiple steps in autophagosome formation in yeast and mammals, including integrating nutrient status via the target of rapamycin (Tor) kinase, recruiting other Atg proteins to the PAS in yeast and forming punctate structures consisting of Atg proteins in mammalian cells.Citation6,Citation7 The Atg1/ULK1 complex also regulates the cycling of Atg9 between autophagosomes and other punctate structures.Citation6 The molecular composition and regulatory mode of the Atg1 complex, however, differ substantially between yeast and mammals.Citation6 In yeast, under growth conditions, Atg13 is hyperphosphorylated in a Tor-dependent manner and dissociates from Atg1, while nutrient depletion results in hypophosphorylation of Atg13, which binds Atg1 with high affinity and activates its kinase activity.Citation8 The Atg1-Atg13 complex further associates with Atg17-Atg31-Atg29 during starvation-induced autophagy.Citation9 In mammalian cells, ULK1 forms a complex with a highly divergent Atg13 homolog, and with RB1CC1/FIP200, homologs of which are widely conserved in eukaryotes but not in S. cerevisiae.Citation10 Formation of the ULK1-ATG13-RB1CC1 complex is not altered by nutrient conditions. mTOR complex 1 (mTORC1) directly interacts with the Atg1/ULK1 complex in a nutrient-dependent manner and mTOR phosphorylates ULK1 and ATG13.Citation11-Citation13 Recent biochemical purification assays showed that ATG101, which has no homolog in S. cerevisiae, stably associates with the ULK1-ATG13-RB1CC1 complex.Citation14,Citation15 Amino acids 112 to 220 of ATG13 directly interact with ATG101.Citation15 ATG101 regulates the stability and basal phosphorylation of ATG13 and ULK1.Citation14,Citation15 The physiological function of ATG101 in the autophagy pathway, however, is still poorly understood.
In this study, we identified and characterized a novel gene, epg-9, which is required for autophagic degradation of a variety of protein aggregates during C. elegans embryogenesis, a process termed aggrephagy. epg-9 mutants exhibit the same autophagy phenotypes as unc-51 (the C. elegans Atg1 homolog) and epg-1 (the C. elegans Atg13 homolog) mutants.Citation16 epg-9 encodes a novel protein with homology to mammalian ATG101. EPG-9 directly interacts with EPG-1/Atg13. Our study provides physiological evidence that EPG-9/ATG101 forms a complex with EPG-1/Atg13 and is essential for the aggrephagy pathway.
Results
Mutations in epg-9 cause defects in degradation of autophagy substrates and in other autophagy-regulated processes
The C. elegans SQSTM1/p62 homolog T12G3.1 is selectively removed by autophagy during embryogenesis.Citation5 In wild-type embryos, t12 g3.1::gfp is weakly expressed and diffusely localized in the cytoplasm (). We performed a genetic screen to isolate mutants with defective degradation of T12G3.1 during embryogenesis.Citation5 One of 62 mutations isolated from ~10,000 genomes was mapped to a new genetic locus, named epg-9. In epg-9 mutants, T12G3.1::GFP and also endogenous T12G3.1, detected by an antibody, were greatly elevated and accumulated into a large number of spherical-shaped aggregates that were dispersed in the cytoplasm (; Fig. S1A and S1B).
Figure 1. Mutations in epg-9 cause defects in the autophagy pathway. Scale bars (whole embryos: 10 μm; inserts: 5 μm) are only shown once, because C. elegans embryos remain the same size during embryogenesis. (A and B) In wild-type embryos, T12G3.1::GFP is weakly expressed and diffuse in the cytoplasm. (A) Nomarski image of the embryo shown in (B). (C) In epg-9 mutant embryos, T12G3.1::GFP is expressed at greatly elevated levels and accumulates into a large number of aggregates. (D and E) SEPA-1 aggregates are absent in wild-type embryos at the comma stage. (D) DAPI image of the embryo shown in (E). (F) A large number of SEPA-1 aggregates are formed in epg-9 mutant comma stage embryos. Comma stage embryos are shown in (D–F). (G–I) T12G3.1 aggregates (G), detected by anti-T12G3.1 antibody, and PGL granules (H), detected by anti-SEPA-1 antibody, are in close proximity but separable (I) in epg-9 mutant embryos. Inserts: magnified view. A ~200 cell stage embryo is shown in (G–I). (J) Survival of epg-9 mutant L1 larvae is reduced compared with wild type under food depletion conditions (log-rank test, p = 0.000). The median survival duration of wild type and epg-9 mutants was 15.000 [95% confidence interval (CI) 14.920–15.080] and 6.000 (95% CI 5.628–6.372) days, respectively. The difference in survival was compared using the Kaplan–Meier method and log-rank tests.
![Figure 1. Mutations in epg-9 cause defects in the autophagy pathway. Scale bars (whole embryos: 10 μm; inserts: 5 μm) are only shown once, because C. elegans embryos remain the same size during embryogenesis. (A and B) In wild-type embryos, T12G3.1::GFP is weakly expressed and diffuse in the cytoplasm. (A) Nomarski image of the embryo shown in (B). (C) In epg-9 mutant embryos, T12G3.1::GFP is expressed at greatly elevated levels and accumulates into a large number of aggregates. (D and E) SEPA-1 aggregates are absent in wild-type embryos at the comma stage. (D) DAPI image of the embryo shown in (E). (F) A large number of SEPA-1 aggregates are formed in epg-9 mutant comma stage embryos. Comma stage embryos are shown in (D–F). (G–I) T12G3.1 aggregates (G), detected by anti-T12G3.1 antibody, and PGL granules (H), detected by anti-SEPA-1 antibody, are in close proximity but separable (I) in epg-9 mutant embryos. Inserts: magnified view. A ~200 cell stage embryo is shown in (G–I). (J) Survival of epg-9 mutant L1 larvae is reduced compared with wild type under food depletion conditions (log-rank test, p = 0.000). The median survival duration of wild type and epg-9 mutants was 15.000 [95% confidence interval (CI) 14.920–15.080] and 6.000 (95% CI 5.628–6.372) days, respectively. The difference in survival was compared using the Kaplan–Meier method and log-rank tests.](/cms/asset/679fc344-4cd2-4829-a49e-4604bda38220/kaup_a_10921163_f0001.gif)
Degradation of other autophagy substrates was next examined in epg-9 mutants. The zygotically synthesized coiled-coil domain-containing protein SEPA-1 is degraded by autophagy, resulting in the presence of SEPA-1 aggregates only in early stage embryos ().Citation17 In epg-9 mutant embryos, SEPA-1 aggregates dramatically accumulated and persisted in late stage embryos and in larvae ( and data not shown). SEPA-1 is required for autophagic degradation of the germline P granule components PGL-1 and PGL-3 in somatic cells.Citation17 In wild-type embryos, PGL-1 and PGL-3 are exclusively localized in germline precursor cells. As observed in other autophagy mutants, PGL-1 and PGL-3 formed a large number of granules that colocalized with SEPA-1 aggregates in epg-9 mutants (Fig. S1D–S1F). These PGL-1-PGL-3-SEPA-1 aggregates, known as PGL granules, were round-shaped and evenly dispersed in the cytoplasm, like T12G3.1 aggregates (; Fig. S1F). Co-immunostaining revealed that PGL granules were in close proximity to, but separable from, T12G3.1 aggregates (). Degradation of other preferential autophagy substrates,Citation17 C35E7.6, ZK1053.4, F44F1.6 and T04D3.2, was also defective in epg-9 mutants (Fig. S1G–S1R). Taken together, our results show that epg-9 is required for degradation of a variety of autophagy substrates.
We further investigated whether epg-9 is involved in other autophagy-regulated processes in addition to aggrephagy.Citation18,Citation19 Optimal survival of L1 larvae under food depletion conditions requires autophagy activity. In the absence of food, epg-9 mutant L1 larvae lived for a mean of 6 d and a maximum of 15 d (), a significant reduction compared with wild-type L1 larvae. This indicates that epg-9 is required for larval survival under starvation conditions. Autophagy mutants grow slowly and show reduced brood size. epg-9 mutant embryos took 36 h more than wild type to develop into young adults. The brood size of wild-type animals is 277 (± 16, n = 6), while epg-9 mutants only gave rise to an average of 74 progeny (± 8, n = 5, p < 0.001, student’s t-test). 52.5% of epg-9 mutant embryos (n = 284) failed to hatch, compared with 1.5% in wild-type animals (n = 197). These results show that epg-9 mutants display multiple defects associated with loss of autophagy activity.
epg-9 mutants show autophagy phenotypes characteristic of unc-51/Atg1 and epg-1/Atg13 mutants
We further examined the expression and distribution of the C. elegans Atg8 homolog, LGG-1, in epg-9 mutants. Compared with wild-type embryos, both LGG-1-I and LGG-1-II (the lipidated form of LGG-1) dramatically accumulated in epg-9 mutants, as shown by an immunoblot assay (). In wild-type embryos, LGG-1 forms punctate structures that mostly appear at the ~100 to 200 cell stage (). In epg-9 mutants, LGG-1 puncta were absent from most embryonic cells but in a few cells they accumulated into large aggregates (). The LGG-1 puncta were absent in atg-3 epg-9 mutants (Fig. S2A–S2D), indicating that their formation in epg-9 mutants requires PE conjugation. The LGG-1 puncta did not colocalize with PGL granules in epg-9 mutants (Fig. S1S–S1U). This characteristic pattern of formation and distribution of LGG-1 puncta is the same as that in mutants of unc-51/Atg1 and epg-1/Atg13 ( and data not shown), but not in other known autophagy mutants.Citation5,Citation20
Figure 2. epg-9 mutants show autophagy phenotypes characteristic of unc-51/Atg1 and epg-1/Atg13 mutants. (A) Western analysis shows the accumulation of both forms of LGG-1-I and LGG-1-II (lipidated form) in epg-9, epg-1 and unc-51 mutants compared with wild-type animals. Actin serves as a loading control. (B and C) LGG-1 forms distinct small punctate structures in a wild-type embryo. (D–G) In epg-9 (D and E), unc-51 (F) and epg-1 (G) mutant embryos, LGG-1 puncta are absent in most cells but forms aggregates in a few cells that are bigger in size and stronger in intensity than those in wild-type embryos. (B and D) DAPI images of the embryos shown in (C and E), respectively. ~200 cell stage embryos are shown in (B–G). (H and I) ATG-9::GFP is diffusely localized in the cytoplasm in a wild-type embryo. (H) DIC image of the embryo shown in (I). (J and K) In unc-51 (J) and epg-9 (K) mutant embryos, ATG-9::GFP accumulates into a large number of punctate structures. Inserts: magnified view. (L) In epg-3 mutant embryos, PGL granules, detected by anti-SEPA-1 antibody, accumulate and form enlarged irregular-shaped clusters. (M) PGL granules are round-shaped and dispersed in the cytoplasm in epg-3; epg-9 double mutants. (N) Immunostaining signals for PGL granules (green), detected by anti-SEPA-1 antibody, and T12G3.1 aggregates (red) are largely overlapping in epg-3 mutant embryos. (O) PGL granules (green) and T12G3.1 aggregates (red) are separable in the epg-3; epg-9 double mutants. (P) LGG-1 puncta are enlarged and accumulate in epg-3 mutant embryos. (Q) LGG-1 puncta are absent in most cells and only accumulate in a few cells in epg-3; epg-9 double mutants. (R) SEPA-1 aggregates (green) and LGG-1 puncta (red) are largely colocalized in epg-3 mutant embryos. (S) SEPA-1 aggregates (green) are separable from the few LGG-1 puncta (red) in epg-3; epg-9 mutant embryos. Separate images for (N, O, R and S) are shown in Figure S2. (T–W) In cup-5 mutants, SEPA-1::GFP accumulates in autolysosomes and the GFP signal is weaker in intensity and the size of the puncta is greater. In cup-5; epg-9 double mutants, distinct SEPA-1 aggregates are formed as in epg-9 single mutants. (T and V) DIC images of the embryos shown in (U and W), respectively.
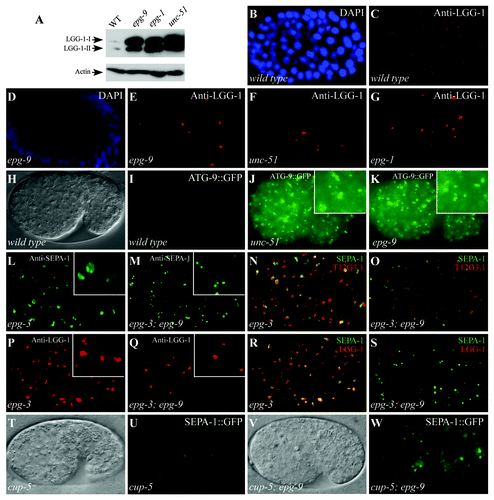
We next determined the distribution pattern of ATG-9::GFP in epg-9 mutants. In wild-type embryos, ATG-9::GFP was diffusely localized in the cytoplasm ().Citation20 In mutants of unc-51 and epg-1, ATG-9::GFP accumulated into a large number of small punctate structures (), a pattern distinct from that in other autophagy mutants.Citation20 We found that ATG-9::GFP accumulated into numerous small, intense puncta in epg-9 mutants (). Taken together, these results indicate that epg-9 mutants exhibit patterns of LGG-1 puncta and ATG-9::GFP puncta characteristic of unc-51 and epg-1 mutants.
epg-9 functions upstream of epg-3 and cup-5 in the aggrephagy pathway
We have previously demonstrated that different autophagy mutants exhibit distinct morphology and distribution pattern of PGL granules, T12G3.1 aggregates and LGG-1 puncta.Citation5,Citation20 To place epg-9 in the aggrephagy pathway, we performed epistasis analysis between epg-9 and other autophagy mutants. epg-3 encodes the C. elegans VMP1 homolog and is involved in the progression of omegasomes to autophagosomes.Citation5 Loss of function of epg-3/VMP1 causes defective degradation of PGL granules and T12G3.1 aggregates.Citation5 In epg-3 mutants, PGL granules and T12G3.1 aggregates formed irregular-shaped clusters and largely colocalized ().Citation5 LGG-1 puncta were also enlarged and colocalized with PGL granules ().Citation5 These genetic phenotypes in epg-3 mutants were suppressed by simultaneous depletion of epg-1 activity (Fig. S2).Citation5 epg-3; epg-9 double mutants also exhibited the same phenotype as epg-9 single mutants; PGL granules and T12G3.1 aggregates were spherical and separable (). LGG-1 puncta were only observed in a few cells and did not colocalize with PGL granules (). Thus, epg-9, like epg-1, acts upstream of epg-3/VMP1 in the aggrephagy pathway.
We also examined the autophagy phenotype in cup-5; epg-9 mutants. cup-5 encodes the C. elegans mucolipin 1 homolog. In cup-5(bp510) mutants, T12G3.1::GFP and SEPA-1::GFP aggregates fail to be removed and are located in the enlarged vacuoles that are labeled by the lysosomal marker NUC-1::cherry.Citation21 T12G3.1::GFP and SEPA-1::GFP aggregates in cup-5 mutants are greater in size and weaker in intensity than those in other autophagy mutants ().Citation21 PGL granules in epg-9 mutant embryos were not colocalized with NUC-1::cherry-labeled lysosomal structures (Fig. S1V–S1X). In cup-5; epg-9 double mutants, the distribution and organization of SEPA-1::GFP aggregates, T12G3.1 aggregates and LGG-1 puncta resembled that in epg-9 single mutants (; Fig. S2). These results indicate that epg-9 acts at a step prior to autolysosome formation.
epg-9 encodes the C. elegans ATG101 homolog and is widely expressed
epg-9 was genetically mapped on chromosome IV, about -7.2. Fosmids from this region were used for transformation rescue experiments. A transgene carrying a single gene, Y69A2AR.7, rescued defective degradation of T12G3.1 aggregates in epg-9(bp320) mutants (). We sequenced cDNAs and found that epg-9 encodes a protein with 260 amino acids. epg-9(bp320) contains a glutamine to an ochre (TAA) stop codon mutation at codon 182 (). Bioinformatic analysis indicates that EPG-9 contains no known motifs. EPG-9 shows 31% identity with human ATG101 with significant homology extending throughout the protein sequence (Fig. S3).Citation14,Citation15 Putative EPG-9 homologs are present in various eukaryotes such as Homo sapiens, Mus musculus, Drosophila melanogaster and Schizosaccharomyces pombe, but are absent in S. cerevisiae.
Figure 3. epg-9 encodes a protein with homology to human ATG101. (A) Cloning of epg-9. epg-9 maps on linkage group IV (LG IV). A transgene containing WRM0632aC02 or Y69A2AR.7 rescued defective degradation of T12G3.1 aggregates in epg-9 mutants. Number of rescued and total examined transgenic lines is indicated. (B) Protein sequence of EPG-9. epg-9(bp320) contains a glutamine to stop codon mutation at amino acid 182 (highlighted in red). (C and D) The integrated transgene carrying the translational epg-9 reporter, bpIs214, is functional in rescuing defective degradation of T12G3.1 aggregates in epg-9 mutants. Compared with , no T12G3.1 aggregates accumulate in epg-9; bpIs214 embryos. (C) Nomarski image of the embryo shown in (D). (E and F) epg-9::gfp is diffusely localized in most cells during embryogenesis. (E) Nomarski image of the embryo shown in (F). (G–J) At postembryonic stages, epg-9::gfp is expressed in pharyngeal muscles and neurons in the head region (G), body wall muscles (arrows) (H) and intestinal cells (I and J). (I) Nomarski image of the animal shown in (J). (K) Interaction between EPG-9 and EPG-1 in a yeast two-hybrid assay using an X-gal assay. EPG-9 was cloned into pPC97 and EPG-1 was cloned into pPC86. V stands for the empty pPC97 or pPC86 vector. (L) EPG-9 directly interacts with EPG-1 and UNC-51 in an in vitro pull-down assay. GST-tagged EPG-9 immobilized on glutathione Sepharose beads was incubated with His-tagged EPG-1 or His-tagged UNC-51(375–856). Proteins retained after extensive washes were detected by western analysis using an anti-His antibody. 20% of the protein used for binding serves as input. (M–P) Expression of epg-1::gfp in wild-type (M and N), epg-9 (O) and unc-51 (P) mutants. The EPG-1::GFP signal is slightly stronger in epg-9 and unc-51 mutants. (M) Nomarski image of the embryo shown in (N).
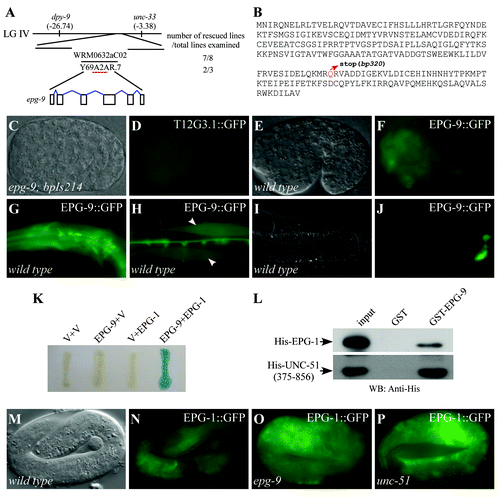
To determine the expression pattern of epg-9, we constructed a translational fusion reporter, which included a 2 kb promoter region and the genomic coding region of epg-9, with gfp inserted at the C terminus of EPG-9. This reporter was functional in rescuing defective degradation of T12G3.1 aggregates in epg-9(bp320) mutants (). EPG-9::GFP was ubiquitously expressed during embryogenesis (). At postembryonic stages, epg-9 was widely expressed, including in pharyngeal muscles, neurons and intestinal cells ( and data not shown).
EPG-9 directly interacts with EPG-1/Atg13 and UNC-51/Atg1
We performed a yeast two-hybrid screen to identify proteins interacting with EPG-9. 45 out of 54 isolated clones corresponded to EPG-1/Atg13 (). We also used EPG-1 as the bait to isolate EPG-1 interacting proteins and 8 out of 17 clones corresponded to EPG-9 (data not shown).
To demonstrate that EPG-9 directly interacts with EPG-1, we performed in vitro GST pull-down assays. Glutathione-S-transferase (GST)-EPG-9 carried on glutathione Sepharose beads was incubated with His-EPG-1. After extensive washes, the retained proteins were separated by SDS-PAGE and analyzed by anti-His antibody. EPG-1 was specifically pulled down by GST-EPG-9, but not by GST (). EPG-9 also directly interacted with UNC-51 in the pull-down assay (). These results suggest that EPG-9 forms a complex with EPG-1/Atg13 and UNC-51/Atg1.
Formation of the EPG-9/EPG-1 complex is not required for the stability of EPG-1 and EPG-9
To determine whether EPG-1/Atg13 is stabilized by forming a complex with EPG-9, we crossed the functional epg-1::gfp reporter into epg-9 and unc-51/Atg1 mutants.Citation16 We found that levels of EPG-1::GFP were not reduced in these mutants (). Levels of EPG-9::GFP displayed no evident reduction in epg-1/Atg13 and unc-51/Atg1 mutants (Fig. S4). Therefore, formation of a complex is not required for the stability of EPG-1 and EPG-9.
Discussion
Here we showed that loss of epg-9 function causes defects in degradation of a variety of autophagy substrates and also in other autophagy-regulated processes. We provided evidence that epg-9 acts at the same genetic step as unc-51 and epg-1 and EPG-9 is likely to form a complex with UNC-51 and EPG-1 in the aggrephagy pathway. First, the autophagy defects in epg-9 mutants are similar to those in unc-51/Atg1 and epg-1/Atg13 mutants. PGL granules and T12G3.1 aggregates are spherical in shape and dispersed in the cytoplasm. In the aggrephagy pathway, epg-9, like epg-1, functions upstream of epg-3/VMP1, which is essential for the progression of omegasomes to autophagosomes.Citation5 LGG-1 puncta are absent in most cells, but accumulate into large punctate structures in a few cells in epg-9, unc-51 and epg-1 mutant embryos.Citation5 epg-9, unc-51 and epg-1 mutants show accumulation of LGG-1-II. Loss of function of these genes may cause accumulation of LGG-1-II on unidentified membrane structures. Second, epg-9 mutants exhibit the distribution pattern of ATG-9::GFP characteristic of unc-51 and epg-1 mutants. Distinct from epg-9 mutant animals, mutants in the essential autophagy genes epg-8 (the C. elegans Atg14 homolog), epg-4 and epg-6 show accumulation of large ATG-9::GFP puncta.Citation20,Citation22 Third, as mutations in epg-1, loss of epg-9 activity abolishes the accumulation of DFCP1::GFP-labeled omegasomes in epg-6 and atg-2 mutants.Citation20 Fourth, epg-9 encodes the mammalian ATG101 homolog, which forms a stable complex with the ULK1-ATG13-RB1CC1/FIP200 complex.Citation14,Citation15 EPG-9 also directly interacts with EPG-1/Atg13 and UNC-51/Atg1. We showed previously that EPG-1 associates with UNC-51/Atg1.Citation16 In addition to defects in the autophagy pathway, dorsal extensions along the lateral hypodermis of DD/VD axons are prematurely terminated or misdirected in unc-51 mutants.Citation23 unc-51 mutants are also uncoordinated and have short bodies.Citation24 epg-9 mutants, like epg-1/Atg13 mutants, have normal locomotion, show no changes in axon migration or in the number of neurons expressing Punc-47::gfp and have a normal body size (Fig. S4). Our genetic analysis strongly argues that EPG-1 and EPG-9 form a complex with UNC-51 that specifically functions in the autophagy pathway.
Our study suggests that the C. elegans Atg1 kinase complex consists of UNC-51/Atg1, EPG-1/Atg13 and EPG-9/ATG101, resembling that in mammals. Unlike in starvation-induced autophagy in other systems, the UNC-51-EPG-1-EPG-9 complex may not be involved in integrating nutrient status via TOR kinase in the aggrephagy pathway during C. elegans embryogenesis. The development of C. elegans embryos relies on the degradation of maternally loaded factors rather than external nutrients. Autophagic degradation of protein aggregates described above occurs at a specific developmental time window and is largely unaffected by TOR signaling.Citation16 In the aggrephagy pathway, the UNC-51-EPG-1-EPG-9 complex may be involved in recruiting other ATG proteins to form autophagosomal membranes surrounding protein aggregates. The role of EPG-9 in the UNC-51-EPG-1 complex remains largely unknown. ATG101 is important for the stability of ATG13 and ULK1 in mammalian cells.Citation14,Citation15 However, levels of EPG-1::GFP and EPG-9::GFP are not reduced in epg-9 and epg-1 mutants, respectively, indicating that formation of a complex is not required for the stability of EPG-1 and EPG-9. The difference could be because C. elegans utilizes a distinct regulatory mode of the Atg1 complex. Alternatively, destabilizing ATG13 and ULK1 by RNAi knockdown of ATG101 in in vitro tissue cultures may not reflect their relationship in vivo. In conclusion, in marked contrast to other Atg proteins, which are well conserved between yeast and human, the Atg1 complex appears to be divergent. The composition and regulatory mode of the Atg1 complex may have evolved to respond to integration of various developmental signals during animal development.
Materials and Methods
Strains
The following strains were used in this work: unc-51(e1189), epg-1(bp414), epg-9(bp320), epg-3(bp405), cup-5(bp510), bpIs211(atg-9::gfp, unc-76), bpIs151(t12 g3.1::gfp, unc-76), bpIs132(c35e7.6::gfp, rol-6), bpIs128(zk1053.4::gfp, rol-6), bpIs175(epg-1::gfp, rol-6), bpIs214(epg-9::gfp, unc-76) and oxIs12(Punc-47::gfp, unc-76).
Characterization and cloning of epg-9
bp320 was identified in the genetic screen for mutants with defective degradation of T12G3.1 aggregates during embryogenesis.Citation5 Three-factor mapping experiments were performed to determine the genetic position of epg-9. From the cross between epg-9 and dpy-9 unc-33 animals, 19 out of 23 Dpy non-Unc animals carried epg-9. Thus, epg-9 locates at genetic position -7.2 on linkage group IV. Fosmids in this region were used for transformation rescue experiments. The fosmid WRM0632aC02 (7 out of 8 transgenic lines) or the PCR product containing Y69A2AR.7 (2 out of 3 transgenic lines) rescued defective degradation of T12G3.1::GFP in epg-9 mutants. The molecular lesions in epg-9(bp320) mutants were determined by sequencing PCR products from the corresponding genomic sequence. By sequencing epg-9 cDNAs, we found that epg-9 contains 6 exons (relative to Y69A2AR, nt 117234 to 117263, 117396 to 117473, 119298 to 119381, 120915 to 121221, 121968 to 122183, 122891 to 122958, the exon 6 is different from the predicted one, which corresponds to nt 123020 to 123120).
Starvation assay
Starvation survival analyses were performed as described.Citation25 Embryos were collected by bleaching old adult hermaphrodites and were then incubated in 3 ml of sterilized water at 20°C. At the indicated time point, an aliquot from each sample tube was placed on a plate seeded with E. coli OP50. After 3 d at 20°C, the number of L4 larvae or adults was counted. The number of animals on day 0 of starvation was as the denominator to calculate the percentage of animals that survived starvation.
Reporter construction
The epg-9::gfp reporter was constructed by a PCR fusion-based approach. The fused PCR products were derived from two overlapping PCR DNA fragments. One contained DNA derived from WRM0632aC02 (nt 9,710 to17,454), which includes a 2 kb promoter region and the entire ORF of epg-9. Another contained gfp and the unc-54 3′UTR from pPD95.67. The PCR products were co-injected with the plasmid expressing wild-type unc-76 into unc-76 animals. The stable transgenic line bpIs214(epg-9::gfp) was obtained after γ-ray irradiation and outcrossed twice.
Yeast two-hybrid assay
Yeast two-hybrid experiments were performed with the ProQuest Two-Hybrid System. Full-length or fragments of EPG-9 and EPG-1(1–228 aa) were fused to the Gal4 DNA binding domain in the vector pPC97, transformed into the yeast host strain MaV203 and then used to screen a C. elegans cDNA library cloned in pPC86. Clones that grew in -Leu-Trp-His medium supplemented with 25 mM 3-aminotriazole (3-AT) were further analyzed.
In vitro pull-down assay
Constructs encoding GST- or His-tagged full length or fragments of EPG-1 and EPG-9 were made by subcloning the corresponding cDNA into pGEX6P-1 (for GST-fusion) and pET-28a(+) (for His-tagging). GST-EPG-9 proteins immobilized on glutathione Sepharose beads were incubated with His-EPG-1 or His-tagged fragments of UNC-51 in binding buffer (25 mM TRIS-HCl (pH 7.6), 150 mM NaCl, 1 mM DTT, 0.5% Triton X-100, 10% glycerol, 1 mM PMSF) overnight at 4°C, then washed five times with 1 ml binding buffer. Bound proteins were separated by SDS-PAGE and analyzed using an anti-His antibody.
Indirect immunofluorescence
Immunofluorescence was performed as described previously.Citation5 Slides were viewed using an epifluorescence microscope or a confocal microscope (Zeiss LSM 510 Meta plus Zeiss Axiovert zoom).
Additional material
Download Zip (4.3 MB)Acknowledgments
We thank members of Dr. Zhang’s laboratory for their helpful comments on the manuscript and Dr. Isabel Hanson for editing work. This work was supported by the National Basic Research Program of China (2010CB835201, 2011CB910100) to H.Z. The research of Hong Zhang was supported in part by an International Early Career Scientist grant from the Howard Hughes Medical Institute.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/autophagy/article/21163
References
- Xie ZP, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol 2007; 9:1102 - 9; http://dx.doi.org/10.1038/ncb1007-1102; PMID: 17909521
- Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol 2009; 10:458 - 67; http://dx.doi.org/10.1038/nrm2708; PMID: 19491929
- Suzuki K, Kubota Y, Sekito T, Ohsumi Y. Hierarchy of Atg proteins in pre-autophagosomal structure organization. Genes Cells 2007; 12:209 - 18; http://dx.doi.org/10.1111/j.1365-2443.2007.01050.x; PMID: 17295840
- Yang Z, Klionsky DJ. Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol 2010; 22:124 - 31; http://dx.doi.org/10.1016/j.ceb.2009.11.014; PMID: 20034776
- Tian Y, Li ZP, Hu WQ, Ren HY, Tian E, Zhao Y, et al. C. elegans screen identifies autophagy genes specific to multicellular organisms. Cell 2010; 141:1042 - 55; http://dx.doi.org/10.1016/j.cell.2010.04.034; PMID: 20550938
- Mizushima N. The role of the Atg1/ULK1 complex in autophagy regulation. Curr Opin Cell Biol 2010; 22:132 - 9; http://dx.doi.org/10.1016/j.ceb.2009.12.004; PMID: 20056399
- Itakura E, Mizushima N. Characterization of autophagosome formation site by a hierarchical analysis of mammalian Atg proteins. Autophagy 2010; 6:764 - 76; http://dx.doi.org/10.4161/auto.6.6.12709; PMID: 20639694
- Kamada Y, Funakoshi T, Shintani T, Nagano K, Ohsumi M, Ohsumi Y. Tor-mediated induction of autophagy via an Apg1 protein kinase complex. J Cell Biol 2000; 150:1507 - 13; http://dx.doi.org/10.1083/jcb.150.6.1507; PMID: 10995454
- Kabeya Y, Kamada Y, Baba M, Takikawa H, Sasaki M, Ohsumi Y. Atg17 functions in cooperation with Atg1 and Atg13 in yeast autophagy. Mol Biol Cell 2005; 16:2544 - 53; http://dx.doi.org/10.1091/mbc.E04-08-0669; PMID: 15743910
- Hara T, Takamura A, Kishi C, Iemura S, Natsume T, Guan JL, et al. FIP200, a ULK-interacting protein, is required for autophagosome formation in mammalian cells. J Cell Biol 2008; 181:497 - 510; http://dx.doi.org/10.1083/jcb.200712064; PMID: 18443221
- Hosokawa N, Hara T, Kaizuka T, Kishi C, Takamura A, Miura Y, et al. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell 2009; 20:1981 - 91; http://dx.doi.org/10.1091/mbc.E08-12-1248; PMID: 19211835
- Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, Cao J, et al. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell 2009; 20:1992 - 2003; http://dx.doi.org/10.1091/mbc.E08-12-1249; PMID: 19225151
- Chang YY, Neufeld TP. An Atg1/Atg13 complex with multiple roles in TOR-mediated autophagy regulation. Mol Biol Cell 2009; 20:2004 - 14; http://dx.doi.org/10.1091/mbc.E08-12-1250; PMID: 19225150
- Hosokawa N, Sasaki T, Iemura SI, Natsume T, Hara T, Mizushima N. Atg101, a novel mammalian autophagy protein interacting with Atg13. Autophagy 2009; 5:973 - 9; http://dx.doi.org/10.4161/auto.5.7.9296; PMID: 19597335
- Mercer CA, Kaliappan A, Dennis PB. A novel, human Atg13 binding protein, Atg101, interacts with ULK1 and is essential for macroautophagy. Autophagy 2009; 5:649 - 62; http://dx.doi.org/10.4161/auto.5.5.8249; PMID: 19287211
- Tian E, Wang FX, Han JH, Zhang H. epg-1 functions in autophagy-regulated processes and may encode a highly divergent Atg13 homolog in C. elegans.. Autophagy 2009; 5:608 - 15; http://dx.doi.org/10.4161/auto.5.5.8624; PMID: 19377305
- Zhang YX, Yan LB, Zhou Z, Yang PG, Tian E, Zhang K, et al. SEPA-1 mediates the specific recognition and degradation of P granule components by autophagy in C. elegans.. Cell 2009; 136:308 - 21; http://dx.doi.org/10.1016/j.cell.2008.12.022; PMID: 19167332
- Sigmond T, Barna J, Tóth ML, Takács-Vellai K, Pásti G, Kovács AL, et al. Autophagy in Caenorhabditis elegans.. Methods Enzymol 2008; 451:521 - 40; http://dx.doi.org/10.1016/S0076-6879(08)03230-8; PMID: 19185738
- Kovács AL, Zhang H. Role of autophagy in Caenorhabditis elegans.. FEBS Lett 2010; 584:1335 - 41; http://dx.doi.org/10.1016/j.febslet.2010.02.002; PMID: 20138173
- Lu Q, Yang PG, Huang XX, Hu WQ, Guo B, Wu F, et al. The WD40 repeat PtdIns(3)P-binding protein EPG-6 regulates progression of omegasomes to autophagosomes. Dev Cell 2011; 21:343 - 57; http://dx.doi.org/10.1016/j.devcel.2011.06.024; PMID: 21802374
- Sun T, Wang XW, Lu Q, Ren HY, Zhang H. CUP-5, the C. elegans ortholog of the mammalian lysosomal channel protein MLN1/TRPML1, is required for proteolytic degradation in autolysosomes. Autophagy 2011; 7:1308 - 15; http://dx.doi.org/10.4161/auto.7.11.17759; PMID: 21997367
- Yang P, Zhang H. The coiled-coil domain protein EPG-8 plays an essential role in the autophagy pathway in C. elegans.. Autophagy 2011; 7:159 - 65; http://dx.doi.org/10.4161/auto.7.2.14223; PMID: 21116129
- Ogura K, Shirakawa M, Barnes TM, Hekimi S, Ohshima Y. The UNC-14 protein required for axonal elongation and guidance in Caenorhabditis elegans interacts with the serine/threonine kinase UNC-51. Genes Dev 1997; 11:1801 - 11; http://dx.doi.org/10.1101/gad.11.14.1801; PMID: 9242488
- Aladzsity I, Tóth ML, Sigmond T, Szabó E, Bicsák B, Barna J, et al. Autophagy genes unc-51 and bec-1 are required for normal cell size in Caenorhabditis elegans.. Genetics 2007; 177:655 - 60; http://dx.doi.org/10.1534/genetics.107.075762; PMID: 17890369
- Kang CH, You YJ, Avery L. Dual roles of autophagy in the survival of Caenorhabditis elegans during starvation. Genes Dev 2007; 21:2161 - 71; http://dx.doi.org/10.1101/gad.1573107; PMID: 17785524