Abstract
The phagophore (also called isolation membrane) elongates and encloses a portion of cytoplasm, resulting in formation of the autophagosome. After completion of autophagosome formation, the outer autophagosomal membrane becomes ready to fuse with the lysosome for degradation of enclosed cytoplasmic materials. However, the molecular mechanism for how the fusion of completed autophagosomes with the lysosome is regulated has not been fully understood. We discovered syntaxin 17 (STX17) as an autophagosomal soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE). STX17 has a hairpin-type structure mediated by two transmembrane domains, each containing glycine zipper motifs. This unique transmembrane structure contributes to its specific localization to completed autophagosomes but not to phagophores. STX17 interacts with SNAP29 and the lysosomal SNARE VAMP8, and all of these proteins are required for autophagosome–lysosome fusion. The late recruitment of STX17 to completed autophagosomes could prevent premature fusion of the lysosome with unclosed phagophores.
The lysosome is a major acidic compartment that contains various hydrolytic enzymes and functions as a degradation site for both endocytosis and autophagy. Autophagy is a major transport pathway by which cytoplasmic materials including cytoplasmic proteins, glycogen, lipids, organelles, and invading bacteria are delivered into the lysosome. The final step of this pathway is achieved by membrane fusion between the autophagosome and lysosome. This fusion step must be strictly regulated; if the phagophore has the ability to fuse with the lysosomal membrane, the partially sequestered cargo will not be delivered into the lysosome lumen.
Specific membrane fusion events in the cell are in general mediated by SNARE proteins, which form a parallel four-helix bundle (composed of Qa-, Qb-, Qc- and R-SNAREs) to bridge two membranes. Indeed, there have been reports that SNARE proteins such as VAMP7, VAMP8 and VTI1 are involved in the autophagosome–lysosome fusion step. However, these SNARE proteins are present primarily on the lysosome or endosome, which precludes a possibility that they function as the autophagosomal SNARE.
Recently, we proposed that the Qa-SNARE STX17 is the autophagosomal SNARE protein based on the following observations: Although STX17 localizes to the ER and mitochondria as well as in the cytosol under nutrient-rich conditions, STX17 translocates to autophagosomes under starvation conditions (). Deletion of the SNARE domain of STX17 does not affect its autophagosomal localization, indicating that this localization is not a secondary result of membrane fusion during autophagosome formation. Depletion of STX17 causes accumulation of undigested autophagosomes, but not phagophores. STX17 interacts with SNAP29 (Qbc-SNARE), which is recruited to autophagosomes upon starvation, and the lysosomal SNARE VAMP8 (R-SNARE) (). Depletion of either SNAP29 or VAMP8 also blocks autophagic flux.
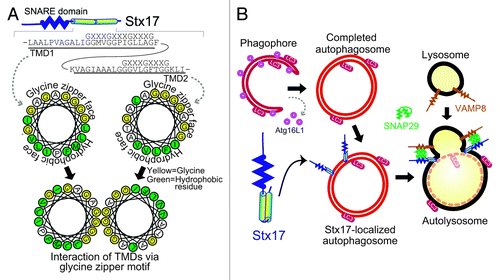
Figure 1. The autophagosomal SNARE STX17 mediates autophagosome–lysosome fusion. (A) STX17 forms a closely packed structure, which is mediated by the two transmembrane domains interacting with each other via the glycine zipper-like motifs, exposing the hydrophobic faces to the hydrophobic part of the lipid bilayer. (B) STX17 is recruited to completed autophagosomes and interacts with SNAP29 and VAMP8, which drives fusion between the outer autophagosomal membrane and lysosomal membrane.
One notable observation is that STX17 does not colocalize with upstream ATG proteins (e.g., ULK1, ATG14, ZFYVE1/DFCP1, WIPI1 and ATG16L1), that serve as markers for the phagophore. Our time-lapse analysis showed that STX17 gradually appears on autophagosomes after LC3 has been recruited. These results suggested that STX17 is recruited to the outer autophagosomal membrane only after completion of autophagosome formation ().
To understand how STX17 is recruited to the autophagosomal membrane, we analyzed several deletion mutants of STX17. Most SNARE proteins are tail-anchored proteins, which have a single transmembrane domain at the C terminus. Tail-anchored proteins are known to post-translationally insert into three organelles—the endoplasmic reticulum (ER), mitochondria and peroxisome. In addition, tail-anchored proteins can be found on other membranes, but they are transported from the ER to their final destinations such as the Golgi complex, endosome and plasma membrane via the membrane trafficking system. The flanking region around the transmembrane domain in tail-anchored proteins is generally considered to determine selective targeting to the ER and mitochondria. Indeed, deletion of the flanking region in STX17 impairs its localization to the ER and mitochondria. However, it does not affect autophagosomal localization of STX17, which is inhibited only when the transmembrane region is removed. Unlike other SNARE proteins, STX17 has two tandem transmembrane domains near the C-terminal end (). The two transmembrane domains of STX17 form a hairpin-type structure with both N- and C-terminal ends on the cytoplasmic side.
The transmembrane domains of STX17 are not as hydrophobic as those of other SNARE proteins, and they contain glycine zipper-like motifs (GXXXGXXXG), which can form closely packed transmembrane helices (). Mutations in the glycine residues on the glycine zipper-like motifs impair localization of STX17 to autophagosomes, but not to the ER and mitochondria. The glycine zipper mutants cannot rescue autophagic activity in STX17-knockdown cells.
Based on these findings, we propose the following model (): Once autophagosome formation is completed, STX17 is recruited to the outer autophagosomal membrane. The two tandem transmembrane domains of STX17, which are important for autophagosomal targeting, form a closely packed hairpin-type structure mediated by the glycine zipper-like motifs. STX17 interacts with cytosolic SNAP29 and lysosomal VAMP8, which induces fusion between the outer autophagosomal membrane and lysosomal membrane.
Several questions arose from these results. Where does STX17 come from? Completed autophagosomes should not be connected to other organelles, suggesting that STX17 may come from the cytosol. In fact, unlike general transmembrane proteins, approximately half of STX17 can be detected in the cytosolic fraction, supporting the idea that cytosolic STX17 translocates to the autophagosomal membrane. If so, what does STX17 recognize on completed autophagosomes? Does insertion of STX17 into autophagosomes require ATP? Is there any chaperone protein(s) that prevents aggregation of STX17 in the cytosol? More detailed analysis of STX17 would provide new insights into the mechanism of autophagosome maturation.
Acknowledgments
This work was supported by the Funding Program for Next Generation World-Leading Researchers, and the Takeda Science Foundation (to N.M.) and grants for a research fellowship of the Japan Society for the Promotion of Science for Young Scientists (to E.I.).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.