Abstract
We have identified in plasma cells a novel ATG5-dependent selective negative control on the secretory pathway, which restricts antibody production, sustaining energy metabolism. Revealing new immune functions, autophagy is required in vivo for antibody responses and to maintain the memory plasma cell compartment.
Plasma cells (PCs) are terminal effectors of the B lymphocyte lineage, responsible for the secretion of all antibodies produced in the organism. PCs form during immune responses upon differentiation of antigen-specific B cells in secondary lymphoid organs (e.g., spleen and lymph nodes). Short-lived PCs yield acute immune responses. In addition, repeated T cell-dependent responses generate long-lived PCs that home in to dedicated bone marrow (BM) niches, potentially able to survive for the individual’s lifetime. These long-lived PCs continuously secrete immunoglobulins (Igs) regardless of antigen presence, providing immediate protection from future infections, thereby constituting the humoral immunological memory.
PCs are professional secretory cells that reshape their own proteome and morphology to secrete massive amounts of antibodies. Intense secretion burdens PCs with metabolic, proteasome, ER and oxidative stress. Hence, the differentiation program is tightly connected to adaptive responses such as the unfolded protein response (UPR), which is activated early during PC differentiation to upgrade the ER secretory capacity and accommodate increased protein load.
In line with a unique role for autophagy in interfacing all these stress pathways, we found autophagy to be strongly induced after B cell activation, as witnessed by higher LC3 processing during ex vivo PC differentiation, increased acidotropic staining, and induction of autophagic transcripts. High autophagy is present not only in ex vivo generated PCs, but also in vivo, both in short-lived and in long-lived BM PCs.
We then assessed the functional relevance of autophagy in PCs using Atg5f/fCD19-Cre mice, which lack the essential autophagic player ATG5 in B cells. Expressing normal spleen B cell populations, and lacking overt B cell phenotypes, these mice are appropriate to investigating PC-associated autophagy. We found that B cells lacking ATG5 can undergo normal PC differentiation both in vivo and in vitro, as they proliferate, increase in size and express PC markers. Thus, to investigate the role of autophagy in this process, we performed an unbiased comparison of the proteome of autophagy-competent and incompetent B cells 3 d after activation with LPS by stable isotope labeling in cell culture (SILAC). Apart from predicted compensatory responses (e.g., LAMP2), we found ER and Ig proteins consistently higher in atg5–/– PCs. In keeping with a role for autophagy in regulating the ER, we documented more abundant ER in the absence of autophagy by electron microscopy, together with further increased UPR activation (well beyond the already high levels that are the hallmark of PCs). Lysosomal protease inhibitors prove sufficient to increase ER proteins in normal differentiating PCs, indicating the existence of a direct ATG5-dependent ER digestion. The abundance of other protein classes (e.g., ribosomes or mitochondria) is not affected in the absence of ATG5, disclosing a selective negative control exerted on the ER by autophagy in PCs.
In cellular models of protein folding diseases, autophagy has been proposed to provide an alternative quality control route in the secretory pathway by clearing polymeric mutant protein aggregates in the ER. In contrast, we found autophagy to physiologically reduce overall ER capacity and its abundant Ig cargo in PCs, which are prototypical professional secretors. Even more surprisingly, radio-metabolic pulse-chase assays show that Ig folding, assembly and secretion are not compromised, but rather are even upgraded in the absence of autophagy, resulting in higher Ig production. The increased Ig output was accounted for by increased de novo Ig expression. We traced back this effect to the increased UPR found in atg5–/– PCs, as we could recapitulate the increased expression of Ig genes and PC transcription factors by treating wild-type differentiating PCs with pharmacological ER stressors to intensify UPR signaling. The data underscore the close relationship existing between adaptive responses and PC differentiation.
The unexpected physiological downregulation of ER capacity and Ig production driven by autophagy defines a novel ATG5-dependent ER homeostatic circuitry. However, it raises the question as to what advantage accrues from such a strong induction of autophagy in PCs. The benefit became evident when we measured intracellular ATP, whose levels dropped in atg5–/– PCs. Thus, the intense metabolic demand of synthesizing antibodies requires functional autophagy, even if this results in lower secretory potential. Indeed, lack of autophagy is toxic to PCs, causing a substantial increase in cell death, as assessed in vitro. Hence, autophagy in PCs serves the dual function of limiting ER capacity and Ig output, while promoting energy metabolism and survival ().
Figure 1. An autophagy-dependent cytoprotective trade-off between Ig synthesis and viability in PC ontogenesis. Autophagy limits ER expansion under pharmacological stress in yeast. We found a similar regulation in mammalian physiology: an ATG5-dependent negative control of the ER in PCs, which discloses a novel mechanism and an unanticipated immune function for autophagy. (A) During PC differentiation, autophagy restricts the secretory capacity, reducing ER stress signaling and the expression of the PC key transcriptional regulators, sXBP-1 (spliced form of XBP-1) and PRDM1/Blimp-1, as well as the production of antibodies, while sustaining ATP and viability. (B) Autophagy is essential in PC ontogenesis. Previously shown to be involved in early B cell development (1), but dispensable for the maintenance of most mature B cells (2), we now prove autophagy necessary for T-independent (3) and T-dependent (4) primary antibody responses. Moreover, we find autophagy dispensable in germinal center (GC) B cells (5), but required to maintain the long-lived memory PC pool in the BM (6). Thus, our study proves autophagy essential across PC ontogenesis.
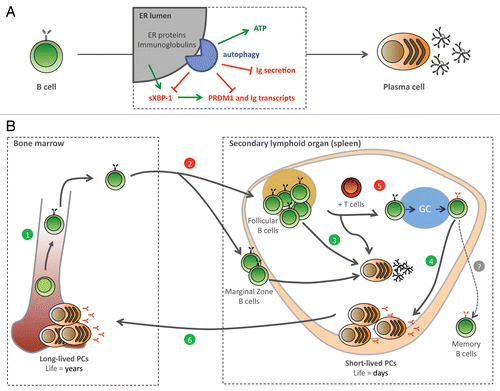
The in vivo functional relevance of autophagy in PCs was established by two key observations. First, we found depressed antibody titers following both T-independent and T-dependent immunizations in Atg5f/fCD19-Cre mice. Second, when we quantified Cre-mediated deletion of Atg5 in sorted BM PCs from old, nonimmunized mice, we found that while the majority of splenic B cells have deleted Atg5, BM PCs from the same mice display normal amounts of nondeleted allele. This efficient selection for autophagy-proficient PCs demonstrated the absolute requirement for autophagy in the formation and/or maintenance of the long-lived PC pool. We also documented a normal germinal center reaction in Atg5f/fCD19-Cre mice. Besides implying that autophagy is dispensable for cognate presentation of soluble antigens by B cells to T cells, this finding locates the requirement for autophagy precisely in PCs ().
It is noteworthy that, one year after T-dependent immunization, Atg5f/fCD19-Cre mice have remarkably fewer antigen-specific long-lived PCs in the BM, further demonstrating that autophagy is a novel determinant of the molecular competence required in the PC memory compartment. Here, autophagy might grant PCs extended survival, as proposed for quiescent progenitors and highly specialized terminally differentiated cell types, such as neurons. These data encourage us to address whether autophagy also plays a role in maintaining the non-Ig-secreting memory B cell compartment.
Finally, our findings provide a framework for identifying novel targets to modulate normal or pathological immune responses, and to fight PC dyscrasias, such as multiple myeloma and light chain amyloidosis, which may rely more on protein homeostatic networks than normal counterparts.
| Abbreviations: | ||
| BM | = | bone marrow |
| Ig | = | immunoglobulin |
| PC | = | plasma cell |
| UPR | = | unfolded protein response |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.