Abstract
Amino acid utilization is important for the growth of the erythrocytic stages of the human malaria parasite Plasmodium falciparum, however the molecular mechanism that permits survival of the parasite during conditions of limiting amino acid supply is poorly understood. We provide data here suggesting that an autophagy pathway functions in P. falciparum despite the absence of a typical lysosome for digestion of the autophagosomes. It involves PfATG8, which has a C-terminal glycine which is absolutely required for association of the protein with autophagosomes. Amino acid starvation provoked increased colocalization between PfATG8- and PfRAB7-labeled vesicles and acidification of the colabeled structures consistent with PfRAB7-mediated maturation of PfATG8-positive autophagosomes; this is a rapid process facilitating parasite survival. Immuno-electron microscopic analyses detected PfRAB7 and PfATG8 on double-membrane-bound vesicles and also near or within the parasite’s food vacuole, consistent with autophagosomes fusing with the endosomal system before being routed to the food vacuole for digestion. In nonstarved parasites, PfATG8, but not PfRAB7, was found on the intact apicoplast membrane and on apicoplast-targeted vesicles and apicoplast remnants when the formation of the organelle was disrupted; a localization also requiring the C-terminal glycine. These findings suggest that in addition to a classical role in autophagy, which involves the PfRAB7-endosomal system and food vacuole, PfATG8 is associated with apicoplast-targeted vesicles and the mature apicoplast, and as such contributes to apicoplast formation and maintenance. Thus, PfATG8 may be unique in having such a second role in addition to the formation of autophagosomes required for classical autophagy.
Introduction
Plasmodium falciparum, the most pernicious causative agent of human malaria, undergoes major remodeling of subcellular structures during development and, moreover, encounters periods of restricted nutritional supply and changes in other conditions, such as temperature and pH, during various parts of its complex life cycle. One regulatory process involved in implementing such cellular differentiation and countering stresses in higher eukaryotes is macroautophagy (henceforth called autophagy).Citation1 A basal level of autophagy is partly responsible for the maintenance of cellular homeostasis by ensuring the removal of damaged, redundant or unwanted cellular components.Citation2 However, autophagy is upregulated in response to diverse environmental and intracellular stresses, most notably during amino acid deprivation, where it replenishes the pool of limiting amino acids. Autophagy involves the sequestration of autophagic cargo into a double-membrane-bound autophagosome prior to targeting to the lysosomal system.Citation3 More than 30 autophagy-related proteins (ATGs) have been identified in yeast and humans defining the central autophagic machinery. Initiation of autophagy is followed by the formation of crescent-shaped phagophores, which is achieved through two complex conjugation pathways employing the ubiquitin-like proteins Atg8 and Atg12.Citation4 In order to drive autophagosome formation from phagophore precursor membranes, Atg8 must become lipidated, resulting in Atg8 association with the outer and inner face of the developing autophagosome. This process, leading to lipidation of Atg8, usually involves first proteolytic truncation by Atg4 to reveal a C-terminal glycine that is conjugated to phosphatidylethanolamine (PE) so that the protein can associate with the autophagosomal membrane.Citation5,Citation6
P. falciparum only encodes a limited number of clearly orthologous Atg proteins (ATG3, ATG4, ATG5, ATG7, ATG8, ATG18) and also has genes potentially encoding ATG1, ATG10, ATG12, and ATG16, but the functionality of these latter proteins in the parasite remains to be assessed.Citation7,Citation8,Plasmodium ATG8 (PfATG8) is unusual as it possesses a free C-terminal glycine, which negates the requirement for proteolytic truncation before lipidation and suggests an unusual form of regulation of PfATG8 function in the parasites. It is reported that PfATG8 binds in vitro to a peptide of a PfATG3 homolog, indicating that an ATG8 conjugation pathway may exist in this parasite.Citation9 Association of PfATG8 with an autophagy-like process in Plasmodium has, however, not been described in detail. Moreover, it has been recently demonstrated that PfATG8 colocalizes with the apicoplast, a unique, non-digestive, chloroplast-like organelle that is essential to the parasite.Citation10 Thus, many questions remain concerning the role of PfATG8.
As autophagy targets cellular cargoes for lytic degradation it is intrinsically linked to the endolysosomal system, although the mechanisms differ with the organism. In higher eukaryotes RAB7 is a key regulator in the trafficking of endosomal material to the lysosomal systemCitation11 and also contributes to lysosome biogenesis.Citation12 RAB7 is necessary for the autophagic process in mammalian cells due to its influence in the maturation of autophagosomes into autolysosomesCitation13 and when deprived of amino acids, ATG8 (known as MAP1LC3/LC3 in mammals) and RAB7 colocalize on autophagosome membranes.Citation14 P. falciparum possesses a family of 11 RABs including a single RAB7 GTPase,Citation15 suggesting that these components of machinery potentially involved in autophagosome maturation exist in malaria parasites. Plasmodium, however, does not contain a classical lysosome and the food vacuole, an acidic organelle in which host cell hemoglobin is digested, is considered the lytic compartment of the parasite.Citation16 The lack of evidence for a functional autophagy pathway in P. falciparum despite the presence of some ATG proteins, and the absence of an apparent lysosome that questions how autophagosomes may be trafficked and digested, prompted us to investigate whether PfATG8-autophagosomes occur in intra-erythrocytic P. falciparum and whether these are trafficked to the food vacuole for subsequent digestion. It was recently reported that under amino acid deprivation P. falciparum enters a dormant phase similar to hibernationCitation17 and our study now provides evidence that such amino acid starvation provokes the fusion of PfATG8- and PfRAB7-positive structures that subsequently enter the food vacuole. This appears to be a parasite-specific adaptation of the canonical autophagy pathway, which could provide the vital amino acids required for parasite survival during amino acid deprivation-induced hibernation. In addition, we show that PfATG8 has a second role relating to the apicoplast. This association requires lipidation of PfATG8 and so constitutes a novel, parasite-specific role for the P. falciparum autophagic machinery potentially important in apicoplast formation and maintenance.
Results
PfATG8 is functional in yeast and is post-translationally modified in Plasmodium
In order to demonstrate that PfATG8 is functionally competent its coding region was expressed in a Saccharomyces cerevisiae strain lacking ScATG8.Citation18 Episomal expression of PfATG8 restored an autophagy competent phenotype, as demonstrated by generation of the mature form of vacuolar aminopeptidase 1 (mAPE1), which is routinely used to show active autophagy following nutrient starvation of transformed yeast cells;Citation18 the negative control LmATG12 did not do this (). In yeast, the C-terminal glycine of Atg8 is revealed by Atg4-mediated cleavage allowing Atg8 to be post-translationally modified with the PE lipid anchor necessary for interaction with autophagosomal membranes.Citation19 Atg4-cleavage of Atg8 gives Atg8-I and eventually leads to formation of PE-lipidated Atg8-II.Citation5,Citation20
Figure 1. PfATG8 complements a Saccharomyces cerevisiae atg8-deficient mutant and is lipidated in Plasmodium. (A) Expression of S. cerevisiae Atg8 (ScAtg8), P. falciparum ATG8 (PfATG8) and Leishmania major ATG12 (LmATG12) in Scatg8∆. Protein extracts were analyzed for the presence of precursor (pAPE1) or mature (mAPE1) vacuolar aminopeptidase by western blotting using anti-APE1 antiserum. N = autophagy not induced, Y = autophagy induced. (B) Extracts of transgenic P. falciparum D10 parasites expressing mCherry-PfATG8∆G (20 µg and 10 µg, respectively) or full-length mCherry-PfATG8 (20 µg) were separated on 6 M urea 15% SDS-PAGE, blotted and probed with anti-mCherry antibody.
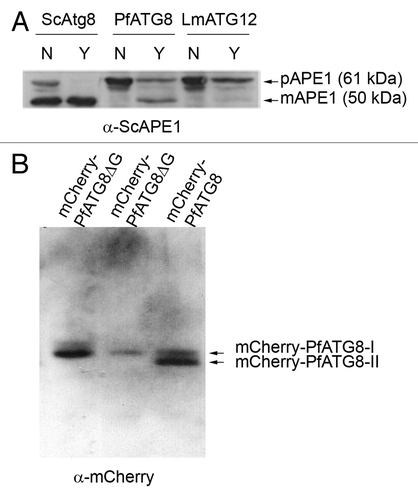
To confirm that PfATG8 is lipidated in vivo, transgenic parasites that expressed mCherry in N-terminal fusion with either PfATG8 or PfATG8ΔG (lacking the C-terminal glycine residue) were generated (Fig. S1). The 810 bp 5′ UTR was used in the expectation of providing a promoter element to control expression of mCherry-PfATG8. In order to assess whether mCherry-PfATG8 is modified by lipidation, urea SDS-PAGE followed by western blotting was performed using anti-mCherry antibodies. mCherry-PfATG8 was present in both lipidated (ATG8-II) and non-lipidated (ATG8-I) forms in the transgenic parasites (; Fig. S1B). In contrast, mCherry-PfATG8ΔG was present only as a single nonlipidated form (), indicating that in vivo lipid modification of PfATG8 is entirely dependent on the C-terminal glycine. Notably the anti-mCherry antibodies only detected one protein band in the parasite lysates expressing PfATG8ΔG with no degradation products detected; this is important for interpreting the subcellular localization of this protein.
PfATG8 colocalizes with the late endosomal marker PfRAB7
It is known that autophagy is particularly important to maintain cellular homeostasis in situations of amino acid starvation.Citation19 When P. falciparum-infected red blood cells were deprived of amino acids for 4 h, the parasites appeared normal, but following 9 h of deprivation morphological changes became evident suggesting that parasite viability may be compromised (). Therefore, parasites were subsequently starved of amino acids for only 4 h in experiments in which the subcellular distribution of PfATG8 and PfRAB7 was followed using anti-peptide antibodies, the specificity of which had been verified (Fig. S2B). Under normal culture conditions the distribution of the two proteins appeared to only partially overlap (), while 4 h deprivation led to an unambiguous colocalization (, mean Pearson’s coefficients from multiple images: upper panel, r = 0.22 ± 0.05, n = 11; lower panel, r = 0.75 ± 0.08, n = 14). A three-dimensional reconstruction of one parasite shows large spheres about 2 µm in diameter containing both PfATG8 and PfRAB7 (). As they were formed upon starvation and labeled with both proteins, they are reminiscent of autophagosomes/autolysosomes that occur in mammalian cells. Electron micrographs of starved parasites revealed that both PfATG8 and PfRAB7 occurred near and within the food vacuole, and also on double-membrane-bound vesicles reminiscent of autophagosomes and/or autolysosomes ().
Figure 2. Amino acid deprivation induces fusion between PfRAB7-positive and PfATG8-positive compartments. (A) Morphological changes observed in P. falciparum-infected erythrocytes in Giemsa stained thin smears 4 h and 9 h after amino acid-deprivation [Zeiss Axio Observer.Z1 microscope with CoolSNAP HQ2 Camera, 100× phase contrast objective (Photometrics)]. (B) Association of PfRAB7 and PfATG8 under normal or amino acid-deprivation conditions using IFA. The distribution of PfRAB7 (red: excitation: 530 to 560 nm and emission 572 to 647 nm; anti-PfRAB7 peptide antibody) and PfATG8 (green: excitation: 450 to 490 nm and emission 500 to 550 nm; anti-PfATG8 peptide antibody) is shown. Nuclei are stained with DAPI (blue: excitation: 340 to 380 nm and emission 450 to 490 nm). The association of PfRAB7 and PfATG8 is shown in white (merge). The images were obtained using a Leica DMI 6000 microscope with MicroMAX-1300Y/HS camera (Princeton Instruments). The Pearson’s coefficient (r) represents the degree of colocalization between PfRAB7 and PfATG8 in the individual images. FV: food vacuole. (C) Volumetric 3D-reconstruction of IFA images using Imaris (Bitplane) corresponding to the Z-stacks taken for the cell in the lowest panel of (B) (r = 0.72) (Leica DMI 6000 microscope with MicroMAX-1300Y/HS camera). The association between PfRAB7 (red) and PfATG8 (green) is shown in white. Nuclei are stained with DAPI (blue). Scale bar: 1 µm.
![Figure 2. Amino acid deprivation induces fusion between PfRAB7-positive and PfATG8-positive compartments. (A) Morphological changes observed in P. falciparum-infected erythrocytes in Giemsa stained thin smears 4 h and 9 h after amino acid-deprivation [Zeiss Axio Observer.Z1 microscope with CoolSNAP HQ2 Camera, 100× phase contrast objective (Photometrics)]. (B) Association of PfRAB7 and PfATG8 under normal or amino acid-deprivation conditions using IFA. The distribution of PfRAB7 (red: excitation: 530 to 560 nm and emission 572 to 647 nm; anti-PfRAB7 peptide antibody) and PfATG8 (green: excitation: 450 to 490 nm and emission 500 to 550 nm; anti-PfATG8 peptide antibody) is shown. Nuclei are stained with DAPI (blue: excitation: 340 to 380 nm and emission 450 to 490 nm). The association of PfRAB7 and PfATG8 is shown in white (merge). The images were obtained using a Leica DMI 6000 microscope with MicroMAX-1300Y/HS camera (Princeton Instruments). The Pearson’s coefficient (r) represents the degree of colocalization between PfRAB7 and PfATG8 in the individual images. FV: food vacuole. (C) Volumetric 3D-reconstruction of IFA images using Imaris (Bitplane) corresponding to the Z-stacks taken for the cell in the lowest panel of (B) (r = 0.72) (Leica DMI 6000 microscope with MicroMAX-1300Y/HS camera). The association between PfRAB7 (red) and PfATG8 (green) is shown in white. Nuclei are stained with DAPI (blue). Scale bar: 1 µm.](/cms/asset/4dc9840a-9349-4e5c-a95d-41affe47d0fd/kaup_a_10925832_f0002.gif)
Figure 3. Immuno-electron microscopy localization of PfATG8 and PfRAB7. (A and B) Immunogold staining of P. falciparum under normal growth conditions and under conditions of amino acid-deprivation (starved) reveals the subcellular localization of PfATG8 (10-nm gold particles; A iii) and PfRAB7 (5-nm gold particles, A iii). (A) PfATG8 and PfRAB7 decorate vesicles (i and insert) and membranous tubules (ii) in the cytoplasm. On some occasions, PfATG8 and PfRAB7 occurred at the periphery of double-membrane-bound organelles (iii, arrowheads). Scale bars: 100 nm. (B) PfATG8 and PfRAB7 associated with large structures corresponding to the food vacuole (FV; i). Costaining of PfATG8 and PfRAB7 is also visible on small double-membrane-bound structures (ii), or very large FV vesicles (iii and iv). Scale bars: 100 nm. (C) Quantitative data to measure the distribution of PfATG8 and PfRAB7 on subcellular structures. For each condition corresponding to normal medium/young FV, normal medium/mature FV, starvation medium/young FV, or starvation medium/mature FV, 35 to 55 parasites have been examined to enumerate the structures containing PfRAB7 alone (white histograms), PfATG8 alone (black histograms) or PfRAB7 and PfATG8 conjointly (gray histograms). Values are expressed in % of total labeled structures for each separate category.
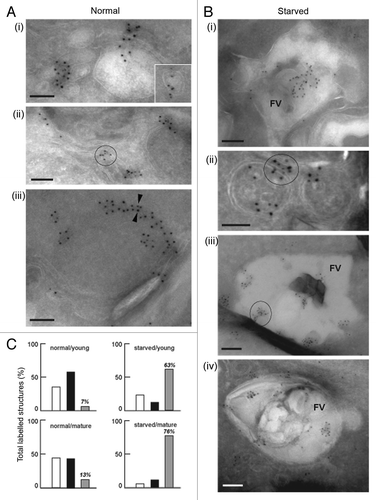
Transgenic parasite lines expressing GFP-PfRAB7 and GFP-PfATG8 were generated (Fig. S2A) and fusion proteins of the expected size and levels were detected using each of anti-GFP and specific anti-PfRAB7 and anti-PfATG8 antibodies (Fig. S2B). The subcellular localization of the tagged proteins as revealed by the anti-GFP antiserum and using antisera against peptides of the proteins were very similar under normal culture conditions (Fig. S2C). In order to analyze whether PfRAB7- and PfATG8-positive vesicles were acidified—a hallmark for the formation of autolysosomes—nonstarved and amino acid-deprived parasites were incubated with the acidotropic dye LysoTracker Red-DND99, which has been reported to label the acidic food vacuole within the parasite.Citation21 Colocalization between PfRAB7 and LysoTracker Red-DND99 was evident to a certain degree even under normal conditions (, upper panel, Pearson’s coefficient from analysis of 13 images, r = 0.56 ± 0.04), which is consistent with PfRAB7 being present in late endosomes, whereas the colocalization between PfATG8 and LysoTracker Red-DND99 was low under the same conditions (, upper panel, Pearson’s coefficient from analysis of 14 images, r = 0.32 ± 0.06). Amino acid deprivation increased the level of PfRAB7 colocalization as well as that of PfATG8-positive structures with LysoTracker Red-DND99, suggesting that an increased level of autophagy was induced by the amino acid deprivation (, lower panels, Pearson’s coefficients from analysis of multiple images, r = 0.82 ± 0.06, n = 20, and r = 0.62 ± 0.07, n = 12, for , respectively). The acidified vesicles appeared to be in close vicinity to the food vacuole or were coincident with it suggesting fusion between the two structures ( and , lower panels). These colocalization data are supported by the immuno-EM data (). In control parasites (), PfATG8 and PfRAB7-labeled vesicles (, i and insert) and membranous tubules (, ii) and PfATG8 also occurred at the periphery of double-membrane-bound organelles (, iii, arrowheads). Upon amino acid deprivation (), an enhanced colocalization of PfATG8 and PfRAB7 with the acidic food vacuole (FV) occurred (, i, iii and iv) and also on small double-membrane-bound structures (, ii). Quantification confirmed that the dual-labeling of structures was considerably greater under amino acid deprivation (, gray bars). Thus the PfATG8- and PfRAB7-labeled acidic compartments have similar features to mature autophagosomes or autolysosomes.
Figure 4. In vivo association between GFP-PfRAB7 and GFP-PfATG8 and acidic compartments. Distribution of GFP-PfRAB7 (A) or GFP-PfATG8 (B) and acidic compartments revealed in red with LysoTracker Red-DND99 is observed under normal and starvation conditions using live cell microscopy [Leica DMI 6000 microscope with MicroMAX-1300Y/HS camera (Princeton Instruments)]. The association of GFP-PfRAB7 and acidic compartments is shown in the merged image (yellow). Comparisons are between parasites at similar stages of development. The Pearson’s coefficient (r) represents the degree of colocalization in the individual images. FV; Food vacuole.
![Figure 4. In vivo association between GFP-PfRAB7 and GFP-PfATG8 and acidic compartments. Distribution of GFP-PfRAB7 (A) or GFP-PfATG8 (B) and acidic compartments revealed in red with LysoTracker Red-DND99 is observed under normal and starvation conditions using live cell microscopy [Leica DMI 6000 microscope with MicroMAX-1300Y/HS camera (Princeton Instruments)]. The association of GFP-PfRAB7 and acidic compartments is shown in the merged image (yellow). Comparisons are between parasites at similar stages of development. The Pearson’s coefficient (r) represents the degree of colocalization in the individual images. FV; Food vacuole.](/cms/asset/b6599b2d-25dd-4c15-8c65-1e081fc7facd/kaup_a_10925832_f0004.gif)
PfATG8 has a second function
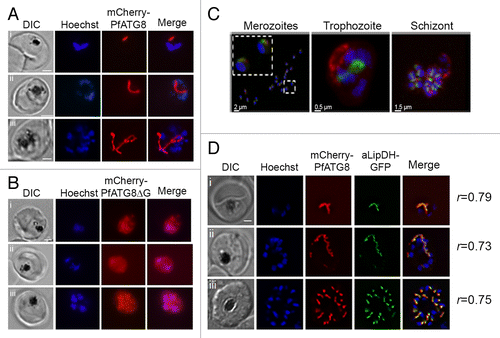
The location of mCherry-PfATG8 was investigated in parasites throughout their intra-erythrocytic life cycle showing that the fluorescent fusion protein associated with distinct structures (); this localization was similar in parasites expressing GFP-PfATG8 (Fig. S2C). The localization of the endogenous protein in addition to associating to structures also showed a punctate distribution (; Fig. S2C and S2D). The association of PfATG8 with subcellular structures was entirely dependent on the C-terminal glycine residue. Removal of the residue (mCherry-PfATG8∆G) led to the protein to have a diffuse, cytosolic distribution (). The diffuse staining pattern made it difficult to know whether the truncated fusion protein still had some association with autophagosomal or other structures. As there was no apparent degradation of the truncated fusion protein in the parasite () that could explain the diffuse, cytosolic staining, the data suggest that localization of PfATG8 to the subcellular structures is dependent on lipid conjugation.
Figure 5. Localization of mCherry-PfATG8 and mCherry-PfATG8∆G. (A) Transgenic mCherry-PfATG8-expressing lines were treated with Hoechst 33258 to stain the nuclei (blue: excitation 365 nm and emission 445 nm) and analyzed by live cell fluorescent light microscopy using an- AxioSkope Mot Plus epifluorescence microscope (Zeiss) with a CCD camera (Hamamatsu) analyzing the distribution of mCherry-PfATG8 (red: excitation 550 and emission 605 nm) which localized to distinct subcellular structures in trophozoite (i and ii), schizont (iii), stage parasites. Images were acquired using OpenLab (Perkin Elmer) and were processed with Image J (NIH) and Photoshop. Scale bar: 2 µm. (B) Transgenic P. falciparum expressing mCherry-PfATG8∆G (red) were stained with Hoechst 33528 (blue) and analyzed as described in (A). Images of trophozoite (i and ii) and schizont (iii) stage parasites are shown. Scale bar: 2 µm. (C) Volumetric 3D-reconstruction of GFP-PfATG8 in vivo (green: excitation 475 to 495 nm and emission 510 to 530 nm) and MitoTracker Red CMXRos (red: excitation548 to 572 nm and emission 590 to 624 nm) in P. falciparum merozoites, trophozoite and schizont using a Zeiss Axio Observer.Z1 microscope with CoolSNAP HQ2 Camera (Photometrics). Nuclei are stained with DAPI (blue: excitation: 381 to 393 nm and emission 420 to 460 nm). Inset in left image shows 2 merozoites on a greater scale. (D) Transgenic P. falciparum expressing both mCherry-PfATG8 and aLipDH-GFP were counterstained with Hoechst 33258 (blue) and analyzed by live cell fluorescent light microscopy using a Delta Vision Core deconvolution microscope (Applied Precision, Inc.) using GFP/FITC filters (excitation 500 nm, emission 560 nm), mCherry filters (excitation 560 nm, emission 630 nm) and DAPI filters (excitation 325 nm, emission 460 nm). Trophozoite (i), early schizont (ii), and late schizont (iii) stages are shown. The Pearson’s coefficients (r) shown next to each image represent the degree of colocalization of mCherry-PfATG8 and aLipDH-GFP in individual images. Scale bar: 2 µm.
Transgenic parasites expressing mCherry-PfATG8 were incubated with wortmannin, the PIK3C3/VPS34 inhibitor that reduces autophagosome formation.Citation22 Wortmannin had no apparent effect on the localization of mCherry-PfATG8, nor did it greatly affect expression or lipidation of the fusion protein (Fig. S3). Thus, modification of PfATG8 and its localization to subcellular structures is either not regulated by PIK3C3 or this enzyme in P. falciparum is relatively insensitive to wortmannin. It has been reported that wortmannin is an effective inhibitor of the parasite’s PIK3C3.Citation23 Lack of sensitivity would, however, not be surprising as it is established that also mammalian and yeast Vps34 orthologs have differing sensitivity to this inhibitor.Citation24
The subcellular structures that PfATG8 associated with under normal growth conditions are similar in shape to both the developing mitochondrion and apicoplast.Citation25 The parasites contain a single mitochondrion and a single apicoplast and both organelles expand throughout intra-erythrocytic development into tubular, branched structures, which eventually segregate into small spherical organelles that are distributed between the daughter merozoites. GFP-PfATG8 and mCherry-PfATG8 transgenic parasites were incubated with MitoTracker CMX-Ros () and MitoTracker Green-FM (Fig. S4A), but no colocalization of PfATG8 with the mitochondrion was observed. This was also confirmed using the mitochondrial protein lipoamide dehydrogenase-GFP (mLipDH-GFP, PF3D7_1232200)Citation26 (Fig. S4B). In contrast, mCherry-PfATG8 partially colocalized with apicoplast lipoamide dehydrogenase-GFP (aLipDH-GFP)-positive structures; aLipDH-GFP is located in the apicoplastCitation26 (, Pearson’s coefficient from analysis of 25 images, r = 0.69 ± 0.07). The apicoplast is present as a single elongated organelle in mature trophozoites (, i). This structure elongates as the parasite develops into a schizont (, ii), before it segments into small organelles, one associated with each new daughter merozoite ( , iii), just before egress.
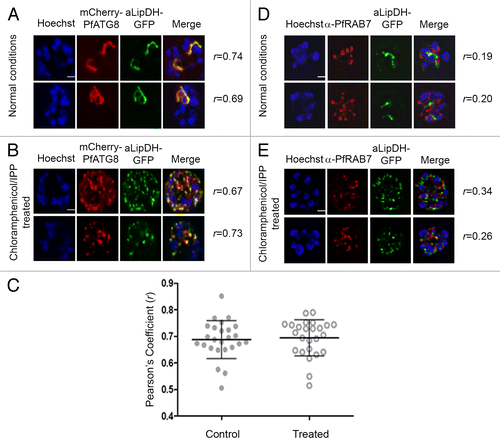
This association with the apicoplast is intriguing and since the organelle is essential for the survival of the intra-erythrocytic stages of Plasmodium, we probed the role of PfATG8 in organelle formation. Antibiotics that inhibit prokaryotic transcription or translation machineries, such as chloramphenicol, specifically block expression of the apicoplast genome and following drug application lead to the loss of the organelle in the second replication cycle of the parasite (known as the delayed death phenotype).Citation27 It was recently shown that addition of isopentenyl-pyrophosphate (IPP) to the culture medium prevented this delayed death phenotype even in the absence of an apicoplast; the parasite was able to survive by salvaging this vital metabolite that is normally generated in the organelle.Citation28 Under these circumstances, the parasite contains multiple vesicles, presumed to have budded from the ER, containing apicoplast-targeted proteins, and also some remnant apicoplast fragments.Citation28 We analyzed the location of mCherry-PfATG8 and the apicoplast marker protein aLipDH-GFP in transgenic parasites expressing both fusion proteins after such chloramphenicol/IPP treatment and showed that both decorated vesicular structures () that have no resemblance to an intact apicoplast (). There was a high degree of colocalization between aLipDH-GFP and mCherry-PfATG8, with there being no difference between treated and control parasites (). These structures may represent ER-budded vesicles trafficking nuclear-encoded apicoplast proteins to the organelle and remnants of the disrupted apicoplast, as discussed later. This function of PfATG8 associated with the apicoplast is entirely independent of PfRAB7, as neither the intact apicoplast nor the vesicles formed when the organelle structure is chemically ablated colocalized with PfRAB7 (). These data strongly support the notion that apart from its canonical function in autophagy PfATG8 has a parasite-specific role in intracellular protein trafficking associated with apicoplast formation and also with homeostasis of the intact apicoplast.
Figure 6. PfATG8, but not PfRAB7, colocalizes with apicoplast-targeted vesicles and apicoplast remnants. (A and B) P. falciparum expressing both mCherry-PfATG8 and aLipDH-GFP were treated with chloramphenicol to chemically disrupt the formation of the apicoplast, and IPP added to the culture medium to maintain viability of the parasites. DMSO control (A) and chloramphenicol treated (B) infected erythrocytes were counterstained with Hoechst 33258. The Pearson’s coefficients (r) show the degree of colocalization in each individual image. (C) Pearson’s coefficient (r) values for parasites coexpressing mCherry-PfATG8 and aLipDH-GFP, either untreated (Control), or treated with chloramphenicol and IPP (Treated). Individual points indicate the r value determined for each parasite analyzed. (D and E) Transgenic parasites expressing aLipDH-GFP were treated as above. Immunofluorescence analyses of untreated controls (D) or chloramphenicol/IPP treated (E) and were performed using anti-PfRAB7 antibodies (red). Hoechst 33258 (blue) was used to stain the nuclei. Scale bar: 2 µm. In all panels, parasites were analyzed using a Delta Vision Core deconvolution microscope (Applied Precision, Inc.) using a 100× objective with the mCherry filter (excitation 560 nm, emission 630 nm) and DAPI filter (excitation 325 nm, emission 460 nm) as indicated. Live cell microscopy was limited to 30 min per sample.
Discussion
This study has provided firm evidence for the occurrence of a functional autophagy pathway in the human malaria parasite P. falciparum. We show that amino acid deprivation induces the formation of PfATG8-positive structures, presumably autophagosomes, which fuse with PfRAB7-positive vesicles (likely late endosomes) prior to fusion with the digestive food vacuole. This is a process resembling the flux of autophagosomes in mammalian cells, but with parasite-specific features. Recently, depriving P. falciparum of essential isoleucine was reported to drive the parasite into a transcriptionally silent hibernation-like state.Citation17 Our observation that PfRAB7- and PfATG8-positive structures fuse within 4 h of amino acid deprivation suggests that autophagy involving autophagosome maturation/delivery is a process that allows the parasite to respond rapidly to lack of nutrients. Moreover, autophagy could provide the vital amino acids required by the parasite to survive the hibernation state.Citation17
The autophagic process functioning in P. falciparum intra-erythrocytic stages must differ in detail from that in mammals, because of the lack of a standard lysosomal network in the parasite combined with its atypical ATG8. It has been revealed by bioinformatics analyses that genes apparently encoding the main components of the ATG8 conjugation pathway are present in the parasite genome and that under normal growth conditions, PfATG8 (PF3D7_1019900), PfATG4 (PF3D7_1417300), PfATG3 (PF3D7_0905700.2) and PfATG7 (PF3D7_1126100) have a similar expression pattern throughout intra-erythrocytic development, with the highest levels of expression after 35 to 40 h post-invasion.Citation29 This suggests that the autophagic machinery is expressed in a concerted manner and that it is highly likely that ATG4, ATG3 and ATG7 are all important for both roles that ATG8 plays in the parasites. The activity of PfATG4 is clearly not required to reveal the C-terminal glycine necessary for lipidation as the amino acid sequence of PfATG8 terminates with this residue, thus regulation of autophagy at this step as in other eukaryotes cannot occur. However, PfATG4 presumably is needed to cleave PfATG8 off the outer face of autophagosomes prior to fusion with the food vacuole, and it is conceivable that it similarly participates in removing ATG8 bound to the apicoplast. Thus autophagy could be partially regulated through the availability of ATG8 ready for lipidation as in higher eukaryotes, but in P. falciparum its availability is controlled through cleavage from lipid association rather than by truncation of its terminal peptide sequence. This is a speculative hypothesis that requires testing in future studies. Initiation and other regulation of autophagy in P. falciparum remains for future studies to explore but one possibility is that signaling pathways involving protein kinases such as ATG1 may play a role. A survey of the three published P. falciparum phosphoproteomes (reviewed in ref. Citation30) has identified in vivo phosphorylation sites not only in PfATG8 but also in PfATG3, PfATG4, PfATG18 (Corresponding GeneID PF3D7_1012900) and PfPI3K (Corresponding Gene ID PF3D7_0515300), which underscore the likely participation of phosphorylation in the regulation.
Despite these differences in the canonical autophagy pathway as it is found in yeast or mammalian cells, our findings revealed that the autophagic process operates in the parasite; this involves the generation of acidified vesicles via the fusion between PfRAB7-positive late endosomes and PfATG8-positive autophagosomes. Immuno-electron microscopy showed that PfRAB7-positive and PfATG8-positive structures appear to fuse with the parasite’s food vacuole, suggesting that Plasmodium possesses an endosomal/lysosomal system in which the food vacuole is the final lytic compartment and thus, in this respect, resembles the lysosomal network typical in higher eukaryotes. These data are in agreement with current knowledge that double-membrane-bound endocytic vesicles are present in the parasite cytosol where they mature and are eventually delivered to the food vacuole, where protein digestion takes place.Citation16 This is also in agreement with a recent review which reports that P. falciparum vesicles are cross-reactive with a heterologous ATG8 antibody,Citation31 although we observed more association with the food vacuole upon starvation rather than a redistribution of the vesicles into the host erythrocyte as claimed in the review. We estimate that under normal growth conditions the number of PfATG8- and PfRAB7-colabeled structures range from 7% in young parasites (rings and young trophozoites) to 13% in more mature stages (late trophozoites and schizonts), which is consistent with constant noninduced autophagy taking place. This basal level of autophagy occurring throughout parasite development might be involved in remodeling of subcellular organelles during intra-erythrocytic growth, and such a role has also been proposed for microneme clearance following sporozoite invasion of hepatocytesCitation32 although a recent paper concludes that autophagy does not occur in liver stages of the parasite.Citation33 A higher level of autophagy is induced upon amino acid deprivation, with many larger structures including the food vacuole becoming labeled with both PfATG8 and PfRAB7. It appears that amino acid starvation does not result in an increased transcription of ATG8 and RAB7,Citation17 which is consistent with our finding that the parasites respond rapidly to amino acid starvation by generating increased numbers of autophagosomes. This suggests that autophagy may be a mechanism that enables the parasite to survive in an apparent hibernation state during amino acid deprivation without the need to adjusting its gene expression.Citation17 In this study we have presented evidence including PfATG8 and PfRAB7 colocalization upon amino acid deprivation along with other features such as the dependence on PfATG8 lipidation for its functional association with subcellular vesicles, which are consistent with autophagy operating in erythrocytic stages of P. falciparum. What remains is to identify the cargo carried within PfRAB7 and PfATG8-positive autophagosomes, to determine the autophagosomal flux using pulse-chase experiments, and to demonstrate that degradation of the cargo within the food vacuole generates the required amino acids; experiments that will be challenging with P. falciparum. Nonetheless, this study by demonstrating the formation of PfATG8-positive double-membrane structures () and autophagosome delivery to the PfRAB7-positive lysosomal machinery (where in the case of P. falciparum the lytic compartment appears to be the food vacuole, and we have shown PfRAB7 and PfATG8-positive structures localize near to it) has started the process of functional dissection of the autophagy pathway in P. falciparum.
Our data show that PfATG8 has a dual role in Plasmodium; on the one hand it functions in the catabolic processes involved in parasite autophagy, but it also strongly associates with the apicoplast implicating a key role for PfATG8 and its lipid conjugation machinery in the biosynthesis and/or maintenance of this organelle (PfATG8 only associates with the apicoplast when its C-terminal glycine is present; ). Such a dual role in both degradative and biosynthetic processes has not been reported for any other Atg8 although interestingly it is now becoming apparent that several autophagy-related proteins do indeed have nonautophagic roles.Citation34 Importantly, PfATG8 associates with vesicles and apicoplast remnants upon chemical ablation of the apicoplast. It is known that nuclear-encoded apicoplast-targeted proteins pass through the ER, where their signal peptide is cleaved and their transit peptide is revealed, directing them to the apicoplast.Citation35 Currently it is thought that vesicles containing apicoplast-targeted proteins bud from the ER and then fuse with the outmost membrane of the apicoplast, although the mechanism trafficking these vesicles between ER and apicoplast has not been elucidated.Citation36 Based on these observations, we hypothesize that PfATG8 has a role in the trafficking of apicoplast-bound vesicles to the developing apicoplast, an idea supported by the association of PfATG8 with aLipDH-positive, PfRAB7-negative vesicles;Citation37,Citation38 this process seems likely to facilitate organelle expansion during parasite growth by provision of key materials contained within the vesicles and also, potentially, providing PE itself as a building block. These ideas could be tested in future experiments using pulse-chase approaches and also markers, once known, for the vesicles trafficking to the apicoplast. The association of PfATG8 with the mature apicoplast also suggests a continued role in the organelle’s maintenance and expansion during intra-erythrocytic development. It is known that a mammalian ortholog of Atg8 known as LC3 interacts with cellular components such as microtubule-interacting proteins via their LC3-interacting domains or LIR domainsCitation39 and it was previously shown that PfATG8 binds to the LIR domain of PfATG3, suggesting that the parasite protein contains structural features that allow protein-protein interactions that may mediate the transport of apicoplast-bound vesicles to their destination.Citation9 This parasite-specific role for PfATG8 may be explained by the presence of a Plasmodium-specific loop in PfATG8,Citation9 which could confer novel effector binding capacities that are absent in mammalian LC3 and yeast Atg8. Another atypical feature of PfATG8 is the lack of the C-terminal region beyond the glycine residue key for lipidation, and this characteristic could well have evolved to facilitate the second role of the protein in apicoplast formation and homeostasis. These features will be explored in future studies to fully understand the function of PfATG8 in trafficking of apicoplast destined vesicles and their role in apicoplast expansion, development and maintenance. The report that some Arabidopsis Atg8 isoforms also lack C-terminal extensions and so act without Atg4 interventionCitation40 raises the intriguing possibility that an analogous process but involving chloroplasts could be operating in plants. The apicoplast is essential for parasite survival, thus the key and parasite-specific role of PfATG8 and its lipidation system in apicoplast function may be exploitable in novel drug discovery and development.
Materials and Methods
Materials
mCherry–pDONR™221, pCHDR-3/4 and pHBlR-3/4 were kind gifts from Professor GI McFadden (University of Melbourne, Melbourne, Australia). Anti-aminopeptidase 1 (APE1) antibodies were a kind gift from Professor DJ Klionsky (University of Michigan, Ann Arbor, MI USA). WR99210 was a kind gift from Jacobus Pharmaceuticals (Princeton, NJ USA). Human blood was obtained from the Glasgow blood transfusion services. All chemicals were purchased from Sigma unless otherwise stated. All restriction enzymes were purchased from New England Biolabs.
Parasite culturing
P. falciparum 3D7 and D10 strains were cultured according to Trager and JensenCitation41 in RPMI 1640 (Life Technologies, 51800-035) supplemented with 0.5% (w/v) Albumax II (11021-29), 200 μM hypoxanthine, 20 μg/ml gentamycin (complete RPMI 1640 medium) in human erythrocytes at a hematocrit between 2.5% and 5%. Parasite cultures were maintained under low oxygen pressure (1% O2, 3% CO2, 96% N2) at 37 °C. To test the effect of wortmannin on the expression and localization of mCherry-PfATG8, transgenic parasites were treated with 10 µM wortmannin for 6 h (the IC50 was determined to be 40 to 70 µM, data not shown) prior to protein extraction and microscopic analysis. 3D7 parasites and GFP-PfRAB7 and GFP-PfATG8 transgenic parasites were deprived of amino acids by culturing them in complete RPMI 1640 medium without amino acids for 4 h and 9 h. The starvation medium contained all components of complete medium (including Albumax II) except for the absence of all amino acids.Citation42 Giemsa-stained thin smears were used to assess parasite morphology before and after starvation.
Generation of transfection constructs
Genomic DNA was isolated from saponin-isolated parasitesCitation43 using the QiaAMP DNA mini kit according to the manufacturers recommendations (Qiagen, 51304). Constructs expressing PfATG8 N-terminally tagged with mCherry were generated using Gateway technology (Life Technologies, 12537-103). PCR fragments were amplified from 100 ng of 3D7 or D10 genomic DNA with Accuprime Pfx SuperMix (Life Technologies, 12344-040) using the following primers: PfATG8-5′ (sense): 5′-GGGGACAACT TTGTATAGAA AAGTTGCGGG AAAAATAAAA AGTTGAACAC-3′; PfATG8-5′ (antisense): 5′-GGGGACTGCT TTTTTGTACA AACTTGCTAT AAATAAAAAT GTAAGTAC-3′; PfATG8 or PfATG8∆G (sense): 5′-GGGGACAGCT TTCTTGTACA AAGTGGTTCC ATCGCTTAAA GACGAAG-3′; PfATG8 (antisense): 5′-GGGGACAACT TTGTATAATA AAGTTGCTTA TCCTAGACAA CTCTCACAAC TATATTC-3′; PfATG8∆G (antisense): 5′-GGGGACAACT TTGTATAATA AAGTTGCTTA TAGACAACTC TCACAACTAT ATTC-3′; where att sites are in bold letters. Gel purified PCR products were used to generate the entry clones PfATG8-5′-pDONR™P4-P1R, PfATG8-pDONR™P2R-P3 and PfATG8∆G-pDONR™P2R-P3 via the BP reaction according to the manufacturer’s recommendations. Sequences were verified (Sequencing Service Dundee). Along with mCherry–pDONR™221, pCHDR-3/4 and pHBlR-3/4,Citation25 the expression vectors PfATGtg8-5′-mCherry-PfATG8-pCHDR-3/4, PfATG8-5′-mCherry-PfATG8∆G-, pCHDR-3/4 and PfATG8-5′-mCherry-PfATG8-pHBlR-3/4 were generated via the LR reaction. The complete coding sequence (CDS) of PfATG8 (375 bp) and PfRAB7 (621 bp) were PCR amplified from cDNA of 3D7 P. falciparum (Fig. S1) and cloned into the AvrII/XhoI site of the P. falciparum expression vector pARL-GFPCitation44 to generate GFP-PfATG8 and GFP-PfRAB7. The primers used were: 5′-TACCCTAGGA TGCCATCGCT TAAAG-3′ (PfATG8 sense); 5′-TAGCTCGAGT TATCCTAGAC AACTCTC-3′ (PfATG8 antisense) and 5′-TTACCTAGGA TGTCAAATAA AAAAAGAACC-3′ (PfRAB7 sense); 5′-CATCTCGAGT TAACAACAAC GACTTTTG-3′ (PfRAB7 antisense). AvrII and XhoI restriction sites are in bold letters. Apicoplast reporter constructs were generated via directional cloning into the vector pHH2.Citation45 PCR fragments encoding amino acids 1–120 of apicoplast dehydrolipoamide dehydrogenase aLipDH (PF3D7_0815900)Citation26 were amplified from 3D7 genomic DNA using the primers: aLipDH (sense): 5′-GCGCAGATCT TAGAATGGTC ATAAGGCAAA ATATTAAAC-3′; aLipDH (antisense): 5′-CGCGCCTAGG GTTCATAATA TTTTGTGTAC TTCC-3′, where BglII and AvrII restriction sites are in bold letters. The fragments were cloned into TOPO Blunt Cloning kit (Life Technologies, K2875-20) and their sequences verified (Sequencing Service Dundee) prior to subcloning into pHH2 to generate aLipDH-GFP Synchronized parasites were transfected according toCitation46,Citation47 and drug resistant parasites were observed in Giemsa-stained thin smears 30 to 60 d later.
Antibody generation
To generate specific anti-PfRAB7 antibodies two peptides (sequences: FALNNQSEQKMYKSR and FLIQASPKDPENFPF) were synthesized, mixed and used to immunize two rats. To generate specific anti-PfATG8 antibodies two peptides (sequences: CERANRSNLPIIEKKK and PSLKDEVSFENRVAE) were synthesized, mixed and used to immunize two guinea pigs (Eurogentec). In addition, full length PfATG8 was expressed in E. coli BLR (DE3) as a N-terminal His-tagged fusion protein using pET28a(+). A PCR fragment was amplified from 3D7 genomic DNA using the primers: PfATG8 (sense) 5′-AGCTAGCCCA TCGCTTAAAG ACGAA-3′ and PfATG8 (antisense) 5′-GCTCGAGTTA TCCTAGACAA CTCTCACA-3′ and cloned into pET28a(+) previously digested with NheI and XhoI to generate His-PfATG8-pET28a(+). The protein was purified using Ni-NTA agarose, separated from contaminating proteins by SDS-PAGE, excised from the gel and used to immunise two rabbits (Eurogentec).
Live cell microscopy
Infected erythrocytes were imaged using four different systems. P. falciparum 3D7 expressing GFP-PfRAB7 and GFP-PfATG8 were observed under a Leica DMI 6000 microscope with MicroMAX-1300Y/HS camera (Princeton Instruments) or a Zeiss Axio Observer.Z1 microscope (Carl Zeiss Ltd.) with CoolSNAP HQ2 Camera (Photometrics). The parasites were analyzed using a 100× phase contrast objective (×1.6) with oil immersion using the DAPI filters (excitation at 340 to 380 nm; emission at 450 to 490 nm or excitation at 381 to 393 nm and emission at 420 to 460 nm, respectively), FITC filters (excitation at 450 to 490 nm; emission at 500 to 550 nm or excitation at 475 to 495 nm and emission at 510 to 530 nm, respectively) and TRITC filters (excitation at 530 to 560 nm; emission at 572 to 647 nm or excitation at 548 to 572 nm and emission at 590 to 624 nm, respectively). Images were acquired with Z- stacks using MetaMorph (Universal Imaging), de-convoluted with Huygens (SVI) and processed with ImageJ (NIH) and Photoshop (Adobe Systems Inc.). mCherry-PfATG8 and mCherry-PfATG8∆G transgenic parasite lines were observed under an AxioSkope Mot Plus epifluorescence microscope (Carl Zeiss Ltd.) with a CCD camera (Hamamatsu Photonics). The filters used were the DAPI filter (Zeiss filter set 49; excitation 365 nm and emission 445 nm) and the rhodamine filter (Zeiss filter set 43HE; excitation 550 and emission 605 nm). Images were acquired using OpenLab (Perkin Elmer) and were processed with Image J and Photoshop. Transgenic parasites expressing both mCherry-PfATG8 and aLipDH-GFP were analyzed using a DeltaVision Core deconvolution microscope (Applied Precision Inc.) with GFP/FITC filters (excitation 500 nm, emission 560 nm), mCherry filters (excitation 560 nm, emission 630 nm) and DAPI filters (excitation 325 nm, emission 460 nm) with a CoolSNAP HQCitation2 camera (Photometrics). Imaging of parasites was performed using a 100× oil immersion, phase contrast objective. Live imaging was limited to a maximum of 30 min per sample to maintain viability of the parasites. DIC images were taken with polarized light. Three to 5 µm Z-stacks were typically taken with 0.2 µm between images. Deconvolution was performed using a conservative ratio method with 10 iterations. Images were processed and analyzed using SoftWoRx image analysis software (Applied Precision Inc.). Episomal expression is be expected to be variable between cells and indeed this was noted in our imaging work, although it was not investigated in detail and our imaging experiments focused on those cells expressing the fusion proteins well.
LysoTracker and MitoTracker staining
GFP-PfRAB7 and GFP-PfATG8 transgenic lines were cultured under standard conditions (complete medium) and under starvation conditions (medium without amino acids) for 4 h. Cells were stained with DAPI (1 μg/ml) and 100 nM LysoTracker Red-DND99 (Life Technologies, L-7528) for 40 min. Cells were washed 2 times with PBS and a third time with Earle’s balanced salt solution containing 11 mM glucose without phenol red before they were observed as detailed above. Observations were limited to 30 min to ensure parasite viability throughout the analyses. Transgenic parasites were also stained with DAPI (1 μg/ml) and 50 nM MitoTracker Red CMXRos (Life Technologies, M-7512) for 15 min at 37 °C to visualize the active mitochondrion. Images were acquired as outlined above.
Apicoplast colocalization
Infected RBC expressing either mCherry-PfATG8, mCherry-PfATG8∆G or both mCherry-PfATG8 and aLipDH-GFP were enriched using LS+ columns and a VarioMacs magnetic separator (Miltenyi Biotec, 130-042-401 and 130-042-302, respectively) according to Patzewitz et al.Citation48 Subsequently, infected RBCs were incubated with Hoechst 33258 (100 μg/ml) for 5 min at 37 °C, washed with Earle’s balanced salt solution, supplemented with 11 mM glucose and then analyzed as detailed above. Observations were limited to 30 min to ensure parasite viability during the analyses. Pearson’s coefficient values were determined to assess the degree of colocalization between mCherry-PfATG8 and aLipDH-GFP using SoftWoRx (Applied Precision).
Apicoplast disruption
Chemical disruption of the apicoplast was performed as described by Yeh and DeRisi.Citation28 In brief, parasites were treated with 50 μM chloramphenicol daily. After 48 h the medium was supplemented daily with 200 μM isopentenyl-pyrophosphate (IPP) and parasites were prepared for microscopic analysis after a total incubation of 96 h and analyzed by light fluorescence microscopy as outlined above.
Immunofluorescence analyses
After fixing cells with cold 100% methanol, slides were washed with PBS before being permeabilized with 0.1% Triton-X 100 in PBS for 5 min. After washing with PBS, slides were blocked in 3% BSA dissolved in PBS for 1 h at room temperature. Slides were then incubated for 1 h with rat anti-PfRAB7 (1:300) and guinea pig anti-PfATG8 (1:3,000) antisera, followed by four washes with PBS before they were incubated with DAPI (1 μg/ml) and AlexaFluor 594-conjugated anti-rat IgG antibodies (1:3,000) and AlexaFluor 488-conjugated anti-guinea pig IgG antibodies (1:6,000), respectively. Slides were mounted with Dako mounting solution (Agilent, CS703) and cells were examined with an epifluorescence microscope (Leica) as outlined above. For the merge, the ImageJ colocalization plug-in was used and the Pearson’s coefficient was calculated using the ImageJ JACoP plug-in. Using images acquired with Z-stacks (of the cell in lower panel of , starvation condition) a volumic 3D-modelization was performed with Imaris (Bitplane). Immunofluorescence was also performed according to the method of Tonkin et al.,Citation49 using 4% paraformaldehyde and 0.075% glutaraldehyde for 30 min to fix parasites followed by permeabilization with 0.1% Triton X-100. Slides were then blocked in 3% BSA overnight at 4 °C before application of rabbit anti-PfATG8 antisera (1: 1,000) for 1.5 h and goat anti-rabbit AlexaFluor 488-conjugated secondary antibodies (1:1,500) for 1 h. Nucleic acids were stained with Hoechst 33258 (15 μg/ml) for 1 min before the slides were mounted with 2.5% DABCO, 50% glycerol. Microscopic analysis was performed using an AxioSkope Mot Plus epifluorescence microscope (Zeiss) with a CCD camera (Hamamatsu). Images were acquired using OpenLab (Perkin Elmer) and were processed with Photoshop.
Western blotting
Protein for western blotting was isolated from asynchronous or synchronized parasitesCitation50 obtained after saponin lysis of infected RBCs.Citation43 Parasite pellets were lysed in PBS containing 1% Triton X-100 in PBS, 5 μg RNase A, 1 mM phenylmethanesulfonylfluoride, 1 mM benzamidine, 2 μg/mL leupeptin, 10 μM E-64, 2 mM 1,10 phenanthroline, 4 μM pepstatin by 3 cycles of freeze/thaw followed by 5 min centrifugation at 16,000 g at 4 °C, the supernatants were used for analysis. Protein concentrations were determined using the Bradford assay with bovine serum albumin as standard.Citation51 Recombinant His-PfATG8 (1 µg) or parasite lysates isolated from D10 wild type or D10 mCherry-PfATG8 or mCherry-PfATG8ΔG expressing lines (10 µg or 20 µg) were separated using 15% SDS-PAGE or 15% SDS-PAGE gels containing 6 M urea before being transferred to nitrocellulose (Fisher, 88018) using a Transblot SD transfer system (Bio-Rad). After blocking at 4 °C overnight with 5% powdered milk, membranes were probed with a rabbit anti-PfATG8 (1:5,000) antiserum and anti-rabbit HRP-conjugated (1:10,000) secondary antibodies. Anti-branched-chain α-ketoacid dehydrogenase E2 subunit antiserum raised in rabbits (PfBCDH, 1:5,000)Citation52 was used as a loading control on western blots showing endogenous PfATG8 or mCherry-PfATG8 expression. Total protein extracts from parasite lines expressing full length mCherry-PfATG8, GFP-ATG8, mCherry-PfATG8∆G or PfRAB7 were probed with mouse anti-mCherry (1:1,000) monoclonal antibodies (Clontech, 632543), anti-GFP antibody (1:1000, 11814460001), rat anti-PfRAB7 (1:1000) or guinea pig anti-PfATG8 (1:1,500) and the respective HRP-conjugated secondary antibodies at 1,3000 or 1:10,000, respectively. Protein extracts from S. cerevisiae were separated on 7.5% SDS-PAGE gels, transferred to nitrocellulose and probed with rabbit anti-APE1 (1:1,000) antibodiesCitation18 and anti-rabbit HRP-conjugated (1:10,000) secondary antibodies. Signals were detected using the Immobilon Western Kit according to manufacturer’s recommendations (Millipore, P36599) or the ECL chemiluminescence kit (Pierce, 32109). Densitometric analyses of western blots were performed using ImageJ (NIH).
Complementation of Saccharomyces cerevisiae.
Full-length PfATG8 was amplified from 3D7 genomic DNA using the primers: PfATG8 (sense) 5′-GCGCGGATCCATGCCATCGC TTAAAGACGA AG-3′ and PfATG8 (antisense) 5′-GCGCCTGCAGTTATCCTAGA CAACTCTCAC AACTATATTC-3′ (restriction sites BamHI and PstI are indicated in bold). The PCR fragment was cloned into pSC-B (StrataClone, UK) and its sequence verified (Sequencing Service Dundee) before it was subcloned into pCM186 (EUROSCARF, P30323) to generate the construct PfATG8-pCM186. S. cerevisiae strain Y03104 (Scatg8∆) was transformed (as in ref. Citation53) with PfATG8-pCM186, and LmATG12-pCM186Citation54 as a negative control. Autophagy in transformed and wild-type (Y0000) S. cerevisiae lines was induced by culturing them in selective SC-medium (7 g/l yeast nitrogen base without amino acids, 1.92 g/l Kasier drop-out supplement without uracil, 20 g/l glucose) or rich YPD-medium (20 g/l peptone, 10 g/l yeast extract, 20 g/l glucose) at 30 °C until OD600 ~1.0. Half the culture was harvested while the other half was washed into induction IND-medium (1.9 g/l yeast nitrogen base without amino acids and ammonium sulfate, 20 g/l glucose, 2% (v/v) glycerol) and further incubated for 16 h at 30 °C. Cells were harvested and pellets resuspended in PBS, 1 mM PMSF, 1 mM benzamidine, 2 μg/ml leupeptin, 10 μM E-64, 2 mM 1,10 phenanthroline, 4 μM pepstatin and protein extracted using a One Shot cell disrupter (Constant Systems) followed by centrifugation at 50,000 g for 45 min at 4 °C. Supernatants were collected, and protein concentrations were determined using the Bradford method.Citation51
Immuno-electron microscopy
P. falciparum-infected erythrocytes (mixed blood stages) were cultivated under normal or amino acid-deprived conditions (see description above) before fixation, first in 4% paraformaldehyde (PFA) in 0.25 M HEPES (pH 7.4) for 1 h at room temperature, then in 8% PFA in the same buffer overnight at 4 °C. Samples were infiltrated, frozen and sectioned (thin sections of 50 to 60 nm thickness) as previously described.Citation55 The sections were immune-labeled with anti-PfATG8 peptide antibody at 1:500 dilution and anti-PfRAB7 peptide antibody at 1:100 dilution in PBS/1% fish skin gelatine, then with IgG antibodies, followed directly by 10 nm (for PfATG8) and 5 nm (for PfRAB7) protein A-gold particles before examination with a Philips CM120 Electron Microscope (Eindhoven) under 80 kV.
| Abbreviations: | ||
| APE1 | = | vacuolar aminopeptidase 1 |
| ATG | = | autophagy-related (gene) |
| ATG | = | autophagy-related (protein) |
| ATG8-I | = | ATG4-cleaved form of ATG8 |
| ATG8-II | = | lipidated form of ATG8 |
| CDS | = | coding sequence |
| GFP | = | green fluorescence protein |
| IPP | = | isopentenyl-pyrophosphate |
| mCherry | = | mCherry fluorescence protein |
| PE | = | phosphatidylethanolamine |
| PfATG8 | = | Plasmodium falciparum ATG8 |
| PfATG8ΔG | = | PfATG8 lacking the C-terminal glycine residue |
| PfATG3 | = | P. falciparum ATG3 |
| aLipDH-GFP | = | apicoplast lipoamide dehydrogenase-GFP |
| mLipDH-GFP | = | mitochondrial lipoamide dehydrogenase-GFP |
| LmATG12 | = | Leishmania major ATG12 |
| mAPE1 | = | mature form of vacuolar aminopeptidase 1 |
| pAPE1 | = | precursor form of vacuolar aminopeptidase 1 |
| ScAtg8 | = | Saccharomyces cerevisiae Atg8 |
| Scatg8∆ | = | Saccharomyces cerevisiae lacking ATG8 |
Additional material
Download Zip (3.5 MB)Acknowledgments
AMT was supported by a BBSRC Doctoral Training Grant (BB/D526329/1); FBR by a Fondation de France PhD fellowship; JCM and GHC by the MRC (G0700127) and UR by the GRK1459 (Deutsche Forschungsgemeinschaft). GL acknowledges support of Inserm, the Cnrs and the Labex ParaFrap ANR-11-LABX-0024. We acknowledge European Community Seventh Framework Programmes FP7 MALSIG (GL) and FP7 N° 242095 (SM) and support by the Wellcome Trust (WT061173MA-SM, WT085349).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/autophagy/article/25832
References
- Devenish RJ, Klionsky DJ. Autophagy: mechanism and physiological relevance ‘brewed’ from yeast studies. Front Biosci (Schol Ed) 2012; 4:1354 - 63; http://dx.doi.org/10.2741/S337; PMID: 22652877
- Klionsky DJ, Baehrecke EH, Brumell JH, Chu CT, Codogno P, Cuervo AM, et al. A comprehensive glossary of autophagy-related molecules and processes (2nd edition). Autophagy 2011; 7:1273-94
- Yang Z, Klionsky DJ. An overview of the molecular mechanism of autophagy. Curr Top Microbiol Immunol 2009; 335:1 - 32; http://dx.doi.org/10.1007/978-3-642-00302-8_1; PMID: 19802558
- Tanida I. Autophagosome formation and molecular mechanism of autophagy. Antioxid Redox Signal 2011; 14:2201 - 14; http://dx.doi.org/10.1089/ars.2010.3482; PMID: 20712405
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 2000; 19:5720 - 8; http://dx.doi.org/10.1093/emboj/19.21.5720; PMID: 11060023
- Tanida I, Ueno T, Kominami E. Human light chain 3/MAP1LC3B is cleaved at its carboxyl-terminal Met121 to expose Gly120 for lipidation and targeting to autophagosomal membranes. J Biol Chem 2004; 279:47704 - 10; http://dx.doi.org/10.1074/jbc.M407016200; PMID: 15355958
- Brennand A, Gualdrón-López M, Coppens I, Rigden DJ, Ginger ML, Michels PA. Autophagy in parasitic protists: unique features and drug targets. Mol Biochem Parasitol 2011; 177:83 - 99; http://dx.doi.org/10.1016/j.molbiopara.2011.02.003; PMID: 21315770
- Duszenko M, Ginger ML, Brennand A, Gualdrón-López M, Colombo MI, Coombs GH, Coppens I, Jayabalasingham B, Langsley G, de Castro SL, et al. Autophagy in protists. Autophagy 2011; 7:127 - 58; http://dx.doi.org/10.4161/auto.7.2.13310; PMID: 20962583
- Hain AU, Weltzer RR, Hammond H, Jayabalasingham B, Dinglasan RR, Graham DR, Colquhoun DR, Coppens I, Bosch J. Structural characterization and inhibition of the Plasmodium Atg8-Atg3 interaction. J Struct Biol 2012; 180:551 - 62; http://dx.doi.org/10.1016/j.jsb.2012.09.001; PMID: 22982544
- Kitamura K, Kishi-Itakura C, Tsuboi T, Sato S, Kita K, Ohta N, Mizushima N. Autophagy-related Atg8 localizes to the apicoplast of the human malaria parasite Plasmodium falciparum. PLoS One 2012; 7:e42977; http://dx.doi.org/10.1371/journal.pone.0042977; PMID: 22900071
- Wang T, Ming Z, Xiaochun W, Hong W. Rab7: role of its protein interaction cascades in endo-lysosomal traffic. Cell Signal 2011; 23:516 - 21; http://dx.doi.org/10.1016/j.cellsig.2010.09.012; PMID: 20851765
- Bucci C, Thomsen P, Nicoziani P, McCarthy J, van Deurs B. Rab7: a key to lysosome biogenesis. Mol Biol Cell 2000; 11:467 - 80; http://dx.doi.org/10.1091/mbc.11.2.467; PMID: 10679007
- Gutierrez MG, Munafó DB, Berón W, Colombo MI. Rab7 is required for the normal progression of the autophagic pathway in mammalian cells. J Cell Sci 2004; 117:2687 - 97; http://dx.doi.org/10.1242/jcs.01114; PMID: 15138286
- Jäger S, Bucci C, Tanida I, Ueno T, Kominami E, Saftig P, Eskelinen EL. Role for Rab7 in maturation of late autophagic vacuoles. J Cell Sci 2004; 117:4837 - 48; http://dx.doi.org/10.1242/jcs.01370; PMID: 15340014
- Quevillon E, Spielmann T, Brahimi K, Chattopadhyay D, Yeramian E, Langsley G. The Plasmodium falciparum family of Rab GTPases. Gene 2003; 306:13 - 25; http://dx.doi.org/10.1016/S0378-1119(03)00381-0; PMID: 12657463
- Deponte M, Hoppe HC, Lee MC, Maier AG, Richard D, Rug M, Spielmann T, Przyborski JM. Wherever I may roam: protein and membrane trafficking in P. falciparum-infected red blood cells. Mol Biochem Parasitol 2012; 186:95 - 116; http://dx.doi.org/10.1016/j.molbiopara.2012.09.007; PMID: 23043991
- Babbitt SE, Altenhofen L, Cobbold SA, Istvan ES, Fennell C, Doerig C, Llinás M, Goldberg DE. Plasmodium falciparum responds to amino acid starvation by entering into a hibernatory state. Proc Natl Acad Sci U S A 2012; 109:E3278 - 87; http://dx.doi.org/10.1073/pnas.1209823109; PMID: 23112171
- Scott SV, Hefner-Gravink A, Morano KA, Noda T, Ohsumi Y, Klionsky DJ. Cytoplasm-to-vacuole targeting and autophagy employ the same machinery to deliver proteins to the yeast vacuole. Proc Natl Acad Sci U S A 1996; 93:12304 - 8; http://dx.doi.org/10.1073/pnas.93.22.12304; PMID: 8901576
- Wang CW, Klionsky DJ. The molecular mechanism of autophagy. Mol Med 2003; 9:65 - 76; PMID: 12865942
- Nakatogawa H, Ichimura Y, Ohsumi Y. Atg8, a ubiquitin-like protein required for autophagosome formation, mediates membrane tethering and hemifusion. Cell 2007; 130:165 - 78; http://dx.doi.org/10.1016/j.cell.2007.05.021; PMID: 17632063
- Bohórquez EB, Chua M, Meshnick SR. Quinine localizes to a non-acidic compartment within the food vacuole of the malaria parasite Plasmodium falciparum. Malar J 2012; 11:350; http://dx.doi.org/10.1186/1475-2875-11-350; PMID: 23088166
- Blommaart EF, Krause U, Schellens JP, Vreeling-Sindelárová H, Meijer AJ. The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur J Biochem 1997; 243:240 - 6; http://dx.doi.org/10.1111/j.1432-1033.1997.0240a.x; PMID: 9030745
- Vaid A, Ranjan R, Smythe WA, Hoppe HC, Sharma P. PfPI3K, a phosphatidylinositol-3 kinase from Plasmodium falciparum, is exported to the host erythrocyte and is involved in hemoglobin trafficking. Blood 2010; 115:2500 - 7; http://dx.doi.org/10.1182/blood-2009-08-238972; PMID: 20093402
- Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annu Rev Biochem 1998; 67:481 - 507; http://dx.doi.org/10.1146/annurev.biochem.67.1.481; PMID: 9759495
- van Dooren GG, Marti M, Tonkin CJ, Stimmler LM, Cowman AF, McFadden GI. Development of the endoplasmic reticulum, mitochondrion and apicoplast during the asexual life cycle of Plasmodium falciparum. Mol Microbiol 2005; 57:405 - 19; http://dx.doi.org/10.1111/j.1365-2958.2005.04699.x; PMID: 15978074
- McMillan PJ, Stimmler LM, Foth BJ, McFadden GI, Müller S. The human malaria parasite Plasmodium falciparum possesses two distinct dihydrolipoamide dehydrogenases. Mol Microbiol 2005; 55:27 - 38; http://dx.doi.org/10.1111/j.1365-2958.2004.04398.x; PMID: 15612914
- Dahl EL, Shock JL, Shenai BR, Gut J, DeRisi JL, Rosenthal PJ. Tetracyclines specifically target the apicoplast of the malaria parasite Plasmodium falciparum. Antimicrob Agents Chemother 2006; 50:3124 - 31; http://dx.doi.org/10.1128/AAC.00394-06; PMID: 16940111
- Yeh E, DeRisi JL. Chemical rescue of malaria parasites lacking an apicoplast defines organelle function in blood-stage Plasmodium falciparum. PLoS Biol 2011; 9:e1001138; http://dx.doi.org/10.1371/journal.pbio.1001138; PMID: 21912516
- Bártfai R, Hoeijmakers WA, Salcedo-Amaya AM, Smits AH, Janssen-Megens E, Kaan A, Treeck M, Gilberger TW, Françoijs KJ, Stunnenberg HG. H2A.Z demarcates intergenic regions of the plasmodium falciparum epigenome that are dynamically marked by H3K9ac and H3K4me3. PLoS Pathog 2010; 6:e1001223; http://dx.doi.org/10.1371/journal.ppat.1001223; PMID: 21187892
- Lasonder E, Treeck M, Alam M, Tobin AB. Insights into the Plasmodium falciparum schizont phospho-proteome. Microbes Infect 2012; 14:811 - 9; http://dx.doi.org/10.1016/j.micinf.2012.04.008; PMID: 22569589
- Sinai AP, Roepe PD. Autophagy in Apicomplexa: a life sustaining death mechanism?. Trends Parasitol 2012; 28:358 - 64; http://dx.doi.org/10.1016/j.pt.2012.06.006; PMID: 22819059
- Coppens I. Metamorphoses of malaria: the role of autophagy in parasite differentiation. Essays Biochem 2011; 51:127 - 36; PMID: 22023446
- Eickel N, Kaiser G, Prado M, Burda PC, Roelli M, Stanway RR, Heussler VT. Features of autophagic cell death in Plasmodium liver-stage parasites. Autophagy 2013; 9:568 - 80; http://dx.doi.org/10.4161/auto.23689; PMID: 23388496
- Subramani S, Malhotra V. Non-autophagic roles of autophagy-related proteins. EMBO Rep 2013; 14:143 - 51; http://dx.doi.org/10.1038/embor.2012.220; PMID: 23337627
- Tonkin CJ, Kalanon M, McFadden GI. Protein targeting to the malaria parasite plastid. Traffic 2008; 9:166 - 75; PMID: 17900270
- Parsons M, Karnataki A, Feagin JE, DeRocher A. Protein trafficking to the apicoplast: deciphering the apicomplexan solution to secondary endosymbiosis. Eukaryot Cell 2007; 6:1081 - 8; http://dx.doi.org/10.1128/EC.00102-07; PMID: 17513565
- Sheiner L, Demerly JL, Poulsen N, Beatty WL, Lucas O, Behnke MS, White MW, Striepen B. A systematic screen to discover and analyze apicoplast proteins identifies a conserved and essential protein import factor. PLoS Pathog 2011; 7:e1002392; http://dx.doi.org/10.1371/journal.ppat.1002392; PMID: 22144892
- Spork S, Hiss JA, Mandel K, Sommer M, Kooij TW, Chu T, Schneider G, Maier UG, Przyborski JM. An unusual ERAD-like complex is targeted to the apicoplast of Plasmodium falciparum. Eukaryot Cell 2009; 8:1134 - 45; http://dx.doi.org/10.1128/EC.00083-09; PMID: 19502583
- Pankiv S, Johansen T. FYCO1: linking autophagosomes to microtubule plus end-directing molecular motors. Autophagy 2010; 6:550 - 2; http://dx.doi.org/10.4161/auto.6.4.11670; PMID: 20364109
- Vierstra RD. The expanding universe of ubiquitin and ubiquitin-like modifiers. Plant Physiol 2012; 160:2 - 14; http://dx.doi.org/10.1104/pp.112.200667; PMID: 22693286
- Trager W, Jensen JB. Human malaria parasites in continuous culture. Science 1976; 193:673 - 5; http://dx.doi.org/10.1126/science.781840; PMID: 781840
- Fennell C, Babbitt S, Russo I, Wilkes J, Ranford-Cartwright L, Goldberg DE, Doerig C. PfeIK1, a eukaryotic initiation factor 2alpha kinase of the human malaria parasite Plasmodium falciparum, regulates stress-response to amino-acid starvation. Malar J 2009; 8:99; http://dx.doi.org/10.1186/1475-2875-8-99; PMID: 19435497
- Umlas J, Fallon JN. New thick-film technique for malaria diagnosis. Use of saponin stromatolytic solution for lysis. Am J Trop Med Hyg 1971; 20:527 - 9; PMID: 4105462
- Struck NS, Herrmann S, Schmuck-Barkmann I, de Souza Dias S, Haase S, Cabrera AL, Treeck M, Bruns C, Langer C, Cowman AF, et al. Spatial dissection of the cis- and trans-Golgi compartments in the malaria parasite Plasmodium falciparum. Mol Microbiol 2008; 67:1320 - 30; http://dx.doi.org/10.1111/j.1365-2958.2008.06125.x; PMID: 18284574
- Crabb BS, Rug M, Gilberger TW, Thompson JK, Triglia T, Maier AG, Cowman AF. Transfection of the human malaria parasite Plasmodium falciparum. Methods Mol Biol 2004; 270:263 - 76; PMID: 15153633
- Crabb BS, Cowman AF. Characterization of promoters and stable transfection by homologous and nonhomologous recombination in Plasmodium falciparum. Proc Natl Acad Sci U S A 1996; 93:7289 - 94; http://dx.doi.org/10.1073/pnas.93.14.7289; PMID: 8692985
- Wu Y, Kirkman LA, Wellems TE. Transformation of Plasmodium falciparum malaria parasites by homologous integration of plasmids that confer resistance to pyrimethamine. Proc Natl Acad Sci U S A 1996; 93:1130 - 4; http://dx.doi.org/10.1073/pnas.93.3.1130; PMID: 8577727
- Patzewitz EM, Wong EH, Müller S. Dissecting the role of glutathione biosynthesis in Plasmodium falciparum. Mol Microbiol 2012; 83:304 - 18; http://dx.doi.org/10.1111/j.1365-2958.2011.07933.x; PMID: 22151036
- Tonkin CJ, van Dooren GG, Spurck TP, Struck NS, Good RT, Handman E, Cowman AF, McFadden GI. Localization of organellar proteins in Plasmodium falciparum using a novel set of transfection vectors and a new immunofluorescence fixation method. Mol Biochem Parasitol 2004; 137:13 - 21; http://dx.doi.org/10.1016/j.molbiopara.2004.05.009; PMID: 15279947
- Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol 1979; 65:418 - 20; http://dx.doi.org/10.2307/3280287; PMID: 383936
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72:248 - 54; http://dx.doi.org/10.1016/0003-2697(76)90527-3; PMID: 942051
- Storm J, Perner J, Aparicio I, Patzewitz EM, Olszewski K, Llinas M, Engel PC, Müller S. Plasmodium falciparum glutamate dehydrogenase a is dispensable and not a drug target during erythrocytic development. Malar J 2011; 10:193; http://dx.doi.org/10.1186/1475-2875-10-193; PMID: 21756354
- Gietz RD, Woods RA. Yeast transformation by the LiAc/SS Carrier DNA/PEG method. Methods Mol Biol 2006; 313:107 - 20; PMID: 16118429
- Williams RA, Woods KL, Juliano L, Mottram JC, Coombs GH. Characterization of unusual families of ATG8-like proteins and ATG12 in the protozoan parasite Leishmania major. Autophagy 2009; 5:159 - 72; http://dx.doi.org/10.4161/auto.5.2.7328; PMID: 19066473
- Fölsch H, Pypaert M, Schu P, Mellman I. Distribution and function of AP-1 clathrin adaptor complexes in polarized epithelial cells. J Cell Biol 2001; 152:595 - 606; http://dx.doi.org/10.1083/jcb.152.3.595; PMID: 11157985