Abstract
Mutations in the GBA gene encoding glucocerebrosidase cause Gaucher disease (GD), the most prevalent of the lysosomal storage disorders (LSDs) and increase susceptibility to Parkinson disease (PD). Clinically the two disorders can present in a similar manner with analogous pathological features, suggesting mechanistic links between the two disease states. An increasing body of evidence implicates defects in quality control pathways in both, and suggests that LSDs, as a group, can be classed as disorders of autophagy. Using a mouse model of type II neuronopathic GD, we observed global defects in cellular quality control pathways in midbrain neurons and astrocytes. Our data suggest that downregulation of autophagy, mitophagy, and the ubiquitin-proteasome system (UPS) results in accumulation of dysfunctional and fragmented mitochondria, insoluble SNCA/α-synuclein deposits and ubiquitinated proteins. These observations show that dysfunction of cellular quality control pathways lead to impaired energy and free radical homeostasis, providing new insights into the mechanisms of neurodegeneration in GD and illuminating the links between GD and PD.
Gaucher disease is a rare inborn error of metabolism. It is the most common of the LSDs and is caused by recessive mutations in the GBA gene, resulting in decreased lysosomal function due to the accumulation of substrate (glucocerebroside). Located within the lysosome, GBA is responsible for hydrolysis of its substrate into glucose and ceramide. GD can be classed into three subsets based on age of onset and the presence of central nervous system abnormalities. Type I (OMIM#230800) is the most common and is defined by the absence of central nervous system irregularities. Type II (OMIM#230900) is classed as acute neuronopathic GD and presents with severe neurological defects including seizures in early childhood, whereas type III (OMIM#2301000) is chronic, with a late onset neurodegenerative phenotype. Whereas type I is currently treatable with enzyme replacement therapy, there is no equivalent treatment for types II and III, as the recombinant enzyme as does cross the blood-brain barrier.
The neurological and pathological symptoms observed in type I GD patients are similar to those displayed in Parkinson disease, including loss of dopaminergic neurons in the substantia nigra, SNCA accumulation, tremors at rest, bradykinesia, and rigidity. Indeed, parkinsonism has been reported in many GD patients, suggesting a mechanistic link between the two disease states. Further supporting this, large, multicenter genome-wide association studies have reported a high incidence of GBA mutations in patients with sporadic PD and an increased risk of developing PD in GBA carriers. While the molecular mechanism underlying GD pathology is poorly defined, the primary defect is thought to be lysosomal, due to glucocerebroside accumulation. As the lysosome is central to the autophagy process, much attention has been given to the possible role of autophagy in progression of the disease.
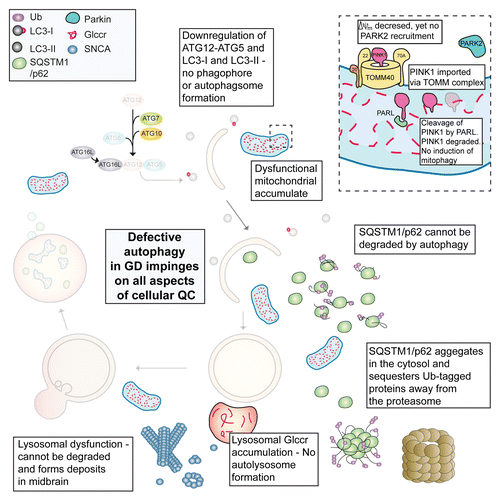
Using an aggressive mouse model of type II neuronopathic GD we found that cellular quality control mechanisms are defective, associated with an inability to turn over the damaged pool of mitochondria in midbrain neurons and astrocytes. As lysosomal function is required for fusion with the autophagosome, this step fails in type II GD due to an accumulation of substrate (). Indeed, the autophagic pathway is downregulated as evidenced by reductions in both LC3-I and LC3-II as well as conjugated ATG12–ATG5 (at the protein, not the mRNA level). Using a GFP-LC3 reporter, we found that LC3-I was unable to be converted to LC3-II, possibly due to the lack of phagophore membranes, presumably preventing conjugation to phosphatidylethanolamine, while autophagic flux was severely reduced. As autophagy is blocked, SQSTM1/p62 accumulates and impinges on the UPS with reduced proteasome activity and accumulation of ubiquitinated proteins, specifically those modified at lysine-48. Insoluble SNCA oligomers fail to be degraded, accumulate and form deposits in the midbrain (). The neurodegenerative pathology here is seen as early as one day postnatal in the mouse brain, and these animal die within 2 weeks of birth.
Figure 1. Defective proteostasis in GD stems from impaired autophagy. This has an impact upon the UPS and mitophagy as well as turnover of aggregated SNCA oligomers. This results in a toxic build-up of damaged proteins and organelles leading to neuronal cell death. Glccr, glucocerebroside.
As mitochondrial dysfunction has long been associated with PD, we investigated whether this was also a feature of GD. The largely fragmented mitochondrial population has a reduced membrane potential (ΔΨm) that is maintained by the reversal of the F1Fo-ATPase (complex V). This phenomenon has also been reported in neurons in which the PD-associated protein PINK1 is depleted, as this is a compensatory mitochondrial mechanism that prevents dissipation of ΔΨm. Mitochondrial-derived reactive oxygen species (ROS) have long been implicated in the pathology of neurodegenerative disorders and are generated by a damaged respiratory chain, primarily at complex I (CI) and to a lesser extent complex III. Exposure of cells to the mitochondrially targeted antioxidant MitoQ10 partially rescues ΔΨm, suggesting the mitochondrial dysfunction observed in this model of GD may be amplified by a feedback cycle involving damage to the respiratory chain by ROS generated at CI.
As mutations in the PARK family of proteins impair mitophagy (mitochondrial specific autophagy) and are associated with familial PD, we investigated whether the defective mitochondrial pool was “marked” for turnover via the PINK1-PARK2 pathway. In spite of the reduced ΔΨm, PARK2 translocation is not observed, suggesting that reversal of the ATPase maintains ΔΨm above the threshold required for PINK1-mediated PARK2 recruitment, thus ensuring that damaged mitochondria are not marked for turnover via mitophagy. This suggests that the inherent mitochondrial compensatory mechanism, reversal of the ATPase, may contribute to the overall pathophysiology in GD.
Overall similar mechanisms appear to engage in the pathophysiology of both GD and PD, with defects in cellular quality control pathways playing central roles in both disease states. The observation of accumulated dysfunctional mitochondria in GD (and PD) highlights the importance of appropriate turnover of this organelle, as failure results in the breakdown of normal energy homeostasis as this indispensible organelle become destructive, leaking ROS and potentially activating the intrinsic apoptotic pathway. Taken together, global defects in proteostasis may underpin the neurodegenerative aspects of both GD and PD. In the end, a pathogenic threshold is crossed as a result of a toxic build-up of misfolded and ubiquitinated protein aggregates (including SNCA) ultimately resulting in neuronal death.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.