Abstract
The role of membrane remodeling and phosphoinositide-binding proteins in autophagy remains elusive. PX domain proteins bind phosphoinositides and participate in membrane remodeling and trafficking events and we therefore hypothesized that one or several PX domain proteins are involved in autophagy. Indeed, the PX-BAR protein SNX18 was identified as a positive regulator of autophagosome formation using an image-based siRNA screen. We show that SNX18 interacts with ATG16L1 and LC3, and functions downstream of ATG14 and the class III PtdIns3K complex in autophagosome formation. SNX18 facilitates recruitment of ATG16L1 to perinuclear recycling endosomes, and its overexpression leads to tubulation of ATG16L1- and LC3-positive membranes. We propose that SNX18 promotes LC3 lipidation and tubulation of recycling endosomes to provide membrane for phagophore expansion.
SNX18 is a member of the SNX9 family, also including SNX33, all containing a phosphoinositide-binding PX domain, and a membrane-remodeling BAR domain, together with an SH3 domain. SNX18 preferentially binds phosphatidylinositol (PtdIns)(4,5)P2 in vitro, which might seem surprising as autophagosome formation requires the class III PtdIns 3-kinase (PtdIns3K) and its product PtdIns3P. However, both mutation of SNX18 to disrupt its membrane-binding ability and depletion of PtdIns(4,5)P2 inhibit SNX18-induced autophagosome formation. The PX-BAR region forms a functional homodimer that has the ability to sense and induce membrane curvature. An amphipathic helix (helix 0) in the low complexity (LC) region of SNX18 is also required for its ability to induce membrane tubulation. We found a time-dependent starvation-induced increase in phosphorylation of Ser233 in helix 0 that could be reversed by refeeding. Interestingly, both nonphosphorylatable and phosphomimicking Ser233 mutations inhibit SNX18-induced membrane tubulation and autophagosome formation, indicating that the membrane-tubulating activity of SNX18 is tightly regulated through cycles of phosphorylation and dephosphorylation, likely to finetune the amount of membranes delivered to forming autophagosomes depending on the current cellular demand for autophagy. Identification of the kinase and phosphatase responsible for this regulation would provide more insight into this.
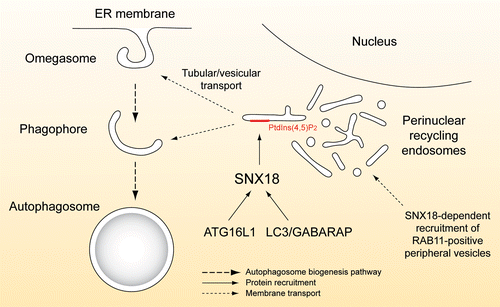
A role for PtdIns(4,5)P2 in autophagy is already established in formation of autophagosome precursors from the plasma membrane, and to further understand the role of SNX18 in autophagy, we sought to determine the origin of the membranes tubulated by SNX18. Multiple origins of autophagosomal membranes have been proposed, including the ER, mitochondria, Golgi, plasma membrane, and recycling endosomes. We detected SNX18 at a perinuclear site and found that markers of recycling endosomes (RAB11 and TFR), as well as ATG16L1, are recruited to this region during starvation. Moreover, membrane tubules containing GFP-LC3, TFR, and ATG16L1 seem to emanate from this perinuclear area upon ectopic expression of SNX18. Knockdown experiments determined the hierarchy between these proteins. SNX18 is required for starvation-induced perinuclear recruitment of RAB11. SNX18 and RAB11 are both required for recruitment of ATG16L1, which again is required for SNX18-induced perinuclear GFP-LC3. Most importantly, depletion of SNX18 prevents recruitment of ATG16L1 to ZFYVE1/DFCP1-positive puncta (omegasomes) and leads to accumulation of WIPI2, indicating that SNX18 functions downstream of ATG14 and the class III PtdIns3K, and upstream of ATG16L1. Consistently, SNX18 overexpression increases the formation of ATG16L1 puncta and Flag-ATG16L1 coimmunoprecipitates with SNX18. These findings are in line with a recent study showing that RAB11 is required for formation of ULK1-, LC3-, and TFR-positive autophagosomes, and that the RAB11-binding protein TBC1D14 relocates from recycling endosomes to the Golgi upon starvation. However, RAB11 has also been implicated in autophagosome maturation and it will be interesting to find out how these apparently different roles of RAB11 in autophagy are regulated.
Previously, ATG14, SH3GLB1/Bif-1, and TBC1D14 have all been implicated in generation or sensing of membrane curvature in autophagy. ATG14 is recruited to highly curved membranes through its BATS domain and recruits the class III PtdIns3K for generation of PtdIns3P on ER membranes. SH3GLB1 colocalizes with ATG5 and LC3, and its BAR domain is required for ATG9 trafficking and fission of Golgi membranes. Overexpression of TBC1D14 induces tubulation of recycling endosomes, thereby inhibiting autophagosome formation. Thus, ATG14, SH3GLB1, TBC1D14, and SNX18 appear to stimulate membrane curvature at different steps of autophagy. Whereas ATG14, and possibly SH3GLB1, is involved in the early membrane remodeling event required for phagophore formation, SNX18 seems to provide membrane input for phagophore expansion, and the extent of such membrane input might be negatively regulated by TBC1D14-mediated tubulation of recycling endosomes. Tubulation of recycling endosomes may also be spatially segregated, since TBC1D14 acts on recycling endosomes at the periphery, whereas SNX18 tubulates perinuclear recycling endosomes. In line with such a model, we found SNX18 to function downstream of ATG14, but the relationship of SNX18 to SH3GLB1 and TBC1D14 has yet to be explored.
We found that endogenous SNX18 co-immunoprecipitates with GFP-LC3B and even more so with the LC3B G120A mutant that cannot be lipidated. Moreover, in vitro translated SNX18 interacts directly with all human LC3/Atg8 family members. The LC3 interaction site was mapped to the sequence WDDEW in the LC region of SNX18. Mutation of the tryptophans in this motif abolish interaction with LC3, and decrease GFP-LC3 puncta formation and recruitment of GFP-LC3 to SNX18-induced membrane tubules. Interestingly, the SNX18 WDDEW motif was previously found to mediate its interaction with adaptor protein 1 (AP1). The role of AP1 in autophagy is not clear, although it has been implicated in autophagosome biogenesis from the trans-Golgi network. The interaction between SNX18 and LC3/GABARAP or AP1 is likely mutually exclusive, and what regulates this interaction remains to be studied. One possible scenario is that SNX18 interacts with AP1 to mediate endosome-to-Golgi transport during conditions of adequate nutrients, and switches to interaction with LC3 during starvation. The observation that both LC3-I and -II are present in the SNX18-ATG16L1-positive membrane fractions suggests that SNX18 might promote LC3 lipidation, but the exact mechanism is yet to be determined.
Finally, we extended our study of mammalian SNX18 to its Drosophila homolog, SH3PX1. By mosaic analysis we found formation of autophagic compartments marked by LysoTracker or mCherry-Atg8a to be inhibited in SH3PX1 RNAi or SH3PX1 mutant cells compared with their wild-type neighbors in fat bodies from third instar larvae, showing that the function of SNX18-SH3PX1 in autophagy is conserved. SH3PX1 also interacts with Drosophila Atg8a, but expression of SH3PX1 is not itself sufficient to induce autophagosome formation, which might be explained by a tighter regulation of autophagy in a multicellular organism than in single cells in culture.
Recent data indicate that various membrane sources are implicated in autophagosome biogenesis, and in line with such a view our results support a model where SNX18 facilitates membrane remodeling of recycling endosomes to feed membrane to the expanding phagophore, formed from ER-associated omegasomes or elsewhere (). Recycling endosomes would be a perfect source of extra membrane in situations of cell crisis, since several vesicular trafficking pathways converge at this site and the further use of the membrane can be efficiently regulated.
Figure 1. Schematic depiction of the proposed role of SNX18 in autophagosome biogenesis. During starvation, RAB11-positive membrane structures, defining TFR-containing recycling endosomes, accumulate at a site near the nucleus in a SNX18-dependent manner. SNX18 further recruits ATG16L1 and LC3/GABARAP to this perinuclear region and through its PtdIns(4,5)P2--binding and membrane remodeling activity SNX18 facilitates generation of LC3/GABARAP-positive carriers that feed membrane onto the growing phagophore.
Acknowledgments
This work was supported by the Molecular Life Science program of the University of Oslo, the Swedish Research Council and the Kempe Foundation.
Disclosure of Potential Conflicts of Interest
The authors declare no conflict of interest.