Abstract
The conjugation of the small ubiquitin (Ub)-like protein Atg8 to autophagic membranes is a key step during the expansion of phagophores. This reaction is driven by 2 interconnected Ub-like conjugation systems. The second system conjugates the Ub-like protein Atg12 to Atg5. The resulting conjugate catalyzes the covalent attachment of Atg8 to membranes. Atg12–Atg5, however, constitutively associates with the functionally less well-characterized coiled-coil protein Atg16. By reconstituting the conjugation of Atg8 to membranes in vitro, we showed that after Atg8 has been attached to phosphatidylethanolamine (PE), it recruits Atg12–Atg5 to membranes by recognizing a noncanonical Atg8-interacting motif (AIM) within Atg12. Atg16 crosslinks Atg8–PE-Atg12–Atg5 complexes to form a continuous 2-dimensional membrane scaffold with meshwork-like architecture. Apparently, scaffold formation is required to generate productive autophagosomes and to deliver autophagic cargo to the vacuole in vivo.
Autophagosomes are de novo generated organelles that sequester cytoplasmic material and deliver it to lysosomal compartments for degradation. Their biogenesis starts with the nucleation of a cup-shaped precursor membrane, termed a phagophore. This structure expands to gradually enclose cytoplasmic material either selectively or nonselectively. Atg8 coordinates phagophore expansion and the amount of Atg8–PE on phagophore membranes correlates with the size of autophagosomes, implying that Atg8 functions as a structural scaffold. In vivo, Atg12–Atg5-Atg16 constitutively localizes to the outer face of the phagophore. Such complexes, however, dissociate from the membrane shortly before or after the phagophore is sealed and an intact autophagosome is generated. A similar observation has been made for the convex pool of Atg8–PE, which is cleaved from the membrane by Atg4 upon completion of the autophagosome.
We reconstituted the conjugation reaction of Atg8 to model membranes in vitro. After its covalent attachment to membranes, Atg8 recruits the Atg12–Atg5 conjugate by directly binding a structural AIM in Atg12. Both conjugates assemble into mobile, oligomeric super-complexes on membranes. Atomic force microscopy revealed that 2 to 4 Atg8–PE-Atg12–Atg5 complexes coalesce into defined particles with a height of 6 nm and an apparent diameter of ~50 nm. Our data showed that a novel noncanonical AIM in Atg12 allows Atg12–Atg5 to reside on membranes in an Atg8–PE-dependent manner independent of the enzymatic activity of Atg12–Atg5, providing an explanation for the colocalization of Atg12–Atg5-Atg16 complexes and Atg8–PE on the outer face of the phagophore. Moreover, Atg16 organizes Atg8–PE-Atg12–Atg5 particles into a continuous 2-dimensional membrane-scaffold with meshwork-like architecture. The length of the edges of this scaffold corresponds to the length of the coiled-coil domain of Atg16, arguing that Atg16 crosslinks such particles into continuous membrane-scaffolds (). On the one hand, the scaffold possesses a lower degree of structural organization than is observed for canonical membrane coats. This provides the scaffold, on the other hand, with structural plasticity, allowing phagophores with such a scaffold on their convex face to capture cytoplasmic cargo of various sizes and shapes. The high plasticity is not without precedent. The structural organization of the canonical coat COPI depends on the curvature of the membrane, providing COPI-coated vesicles with the capacity to accommodate cargo of different sizes.
Figure 1. Speculative cartoon representation of segregated Atg8-functions. (A) Atg8 recognizes an AIM in Atg12, thereby recruiting the Atg12–Atg5-Atg16 complex to membranes. Together with Atg12–Atg5 conjugates Atg8–PE forms oligomeric particles comprising 2 to 4 Atg8–PE-Atg12–Atg5 complexes. Atg16 crosslinks these particles by forming antiparallel oligomers. (B) The scaffold associates at the convex face of autophagosomes. Atg8–PE also localizes to the concave face, where it tethers cargo such as mitochondria to the phagophore by interacting with cargo-specific receptors, for example Atg32.
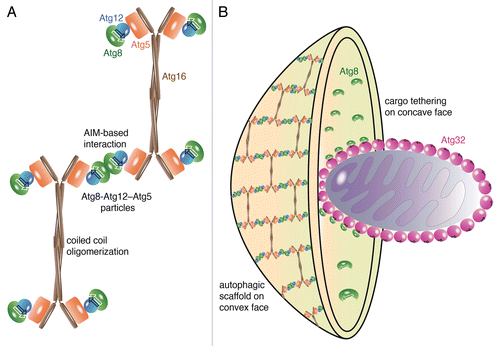
Despite its lower degree of structural organization, the autophagic membrane scaffold possesses some interesting similarities to canonical membrane coats. Canonical membrane coats are composed of building blocks that self-organize into 10-nm (COPII), 12-nm (clathrin), and 14-nm (COPI) thick protein layers, which compares to the 8-nm thick autophagic membrane scaffold. Like canonical coats, the autophagic scaffold is composed of a cargo receptor-binding subunit (Atg8–PE) and a coat component (Atg12–Atg5-Atg16). The edges of the clathrin- and COPII-lattices are composed of antiparallel arranged α-solenoids of the triskelion-leg and of Sec31, respectively. The edges of the autophagic scaffold comprise antiparallel arranged Atg16-coiled coil domains. With a vertex-to-vertex distance of 17 nm, the edge-length of the autophagic scaffold is similar to that in clathrin- (18.5 nm) and COPII-lattices (30 nm). Thus, structurally, this autophagic scaffold and canonical coats appear to be closely related. Functionally, however, scaffold formation (convex phagophore-face) and cargo-binding (concave phagophore-face) functions of Atg8–PE are spatially segregated, whereas both activities are spatiotemporally coordinated and interdependent in canonical membrane coats. Spatial segregation is achieved by competitive binding of cargo receptors, such as Atg32, and Atg12 to Atg8–PE. As a consequence, cargo receptors sequester Atg8–PE from the membrane-scaffold, suggesting that the interaction of Atg8–PE with cargo is involved in defining the concave face of the phagophore while the scaffold is retained on and thereby defines its convex face (). The crucial function of the autophagic scaffold during phagophore expansion is supported by in vivo data; expression of an Atg16 mutant, which does not support scaffold formation in vitro, induces the formation of enlarged but unproductive autophagosomes with prolonged lifetimes in vivo.
The association of Atg12–Atg5-Atg16 and Atg8 to the outer face of the phagophore appears to be temporally coordinated and interdependent. Both are recruited to autophagic membranes during phagophore expansion, but are released upon its completion. Atg8 is recycled by proteolytic cleavage of the amide-bond between its C-terminus and PE. The autophagic protease Atg4 catalyzes this recycling reaction both in vivo and in vitro. Interestingly, the human homolog of Atg4 possesses a LIR (LC3-interacting region) motif that binds the human Atg8-homolog LC3 independently of its catalytic site. Our data showed that yeast Atg4 competes with Atg12 for Atg8–PE binding, suggesting that Atg8 recognizes an AIM in Atg4. As a result, Atg4 sequesters Atg8 from scaffolds in a similar manner as has been observed for the cargo receptor Atg32. Thus Atg4 not only recycles Atg8 by cleaving it from autophagic membranes, it also releases Atg12–Atg5-Atg16 by its competitive interaction with Atg8–PE.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
