Abstract
Like other selective autophagy pathways, the selective autophagy of peroxisomes, pexophagy, is controlled by receptor protein complexes (RPCs). The pexophagic RPC in Pichia pastoris consists of several proteins: Pex3 and Pex14 ligands in the peroxisomal membrane, Atg30 receptor, Atg11, and Atg17 scaffolds, and the phagophore protein Atg8. Recently, we identified a new component of the pexophagic RPC, Atg37, which is involved in the assembly of this complex. Atg37 is an integral peroxisomal membrane protein (PMP) that binds Pex3 and Atg30, but not Pex14 or Atg8. In the absence of Atg37, the recognition of Pex3 and recruitment of Atg17 by Atg30 are normal. However, the recruitment of Atg11 is severely affected suggesting that the role of Atg37 is to facilitate the Atg30-Atg11 interaction. Palmitoyl-CoA competes with Atg30 for the acyl-CoA binding domain of Atg37 in vitro and might regulate the dynamics of the pexophagic RPC in vivo. The human counterpart of Atg37, ACBD5, also localizes to peroxisomes and is specifically required for pexophagy. Therefore, it is tempting to speculate that ACBD5/ATG37 regulates the assembly of the pexophagic RPC in mammalian cells.
Like the P. pastoris pexophagy receptor, Atg30, Atg37 binds the PMP Pex3. Atg37 and Pex3 co-immunoprecipitate both before and during pexophagy, cofractionate with membrane organelles and colocalize on the peroxisomal membrane. These 2 proteins also bind each other directly in vitro. This binding must occur between the cytosolic portions of the 2 proteins, which were used for the experiments in vitro. The acyl-CoA binding site (amino acids Y40 and K44) of Atg37 is not required for Atg37-Pex3 binding. The co-immunoprecipitation of the 2 proteins was even stronger in the absence of Atg30, suggesting that Atg30 and Atg37 might compete for Pex3 and bind different molecules of Pex3 in the peroxisomal membrane.
The role of Atg37-Pex3 binding is still unclear. Unlike Atg30, Atg37 has a well-distinguished C-terminal transmembrane domain, which anchors the protein in the peroxisomal membrane and exposes the N-terminal acyl-CoA binding domain to the cytosol. The transmembrane domain of Atg37 is probably sufficient to support the localization of the protein in the peroxisomal membrane, once it is there. The delivery of Atg37 to the peroxisomal membrane might happen either via direct insertion of the protein from the cytosol or via the ER-to-peroxisome trafficking pathway. In either case, the Atg37-Pex3 interaction could be instrumental in the delivery of Atg37 to the peroxisome, as Pex3 is involved in both routes of PMP recruitment in different model systems.
According to the RPC model, the receptor plays a central role in the RPC by establishing the interactions with ligands, scaffolds, and phagophore proteins. Consistent with this model, Atg30 recruits Atg37 to the pexophagic RPC at the peroxisome cluster periphery. Indeed, in the absence of Atg30, Atg37 mislocalizes to the middle of the peroxisome cluster. P. pastoris Atg30 and Atg37 co-immunoprecipitate and bind each other directly when introduced into another yeast, Saccharomyces cerevisiae, which does not have homologs of these 2 proteins. The proteins also bind each other directly in vitro. Interestingly, the acyl-CoA binding site of Atg37 is not only essential to bind palmitoyl-CoA, but is also required for the Atg30-Atg37 interaction. Moreover, Atg30 and palmitoyl-CoA compete for Atg37 in vitro. It will be interesting to see if palmitoyl-CoA is able to regulate the Atg30-Atg37 interaction in vivo. The molecular dissection of the acyl-CoA binding domain and following the mutants affected only in palmitoyl-CoA, but not Atg30, binding would be required to address the specific role of palmitoyl-CoA in pexophagy.
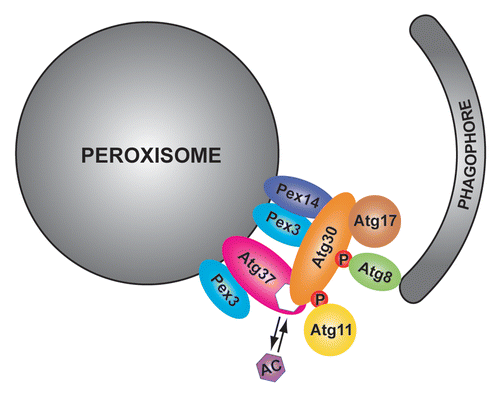
As to the role of Atg37, we found that it is required for the assembly of the pexophagic RPC at a step of the recruitment of Atg11 (). In the absence of Atg37, Atg30 is able to recognize Pex3, and recruit Atg17, but is unable to recruit Atg11, leading to mislocalization of this protein to the vacuolar membrane. Since Atg11 is a major autophagic scaffold during pexophagy in P. pastoris, this causes a major pexophagy defect. However, if Atg11 recruitment was the only role of Atg37, phagophore formation would probably be normal, as in the Atg11-binding site mutant of Atg30. On the contrary, the atg37∆ mutant displays a severe defect of phagophore formation indicating that Atg37 might play an additional role in the pexophagic RPC. We speculate that Atg37 might also regulate Atg30-Atg8 binding, which is required for efficient phagophore formation. If Atg37 affected the phosphorylation of Atg30 at the Atg8- and Atg11-binding sites, it would explain the phagophore formation and pexophagy defects of atg37∆ cells due to the inability of Atg30 to recruit Atg8 and Atg11, respectively. Future studies will surely provide further clues of how Atg37 regulates the assembly of the pexophagic RPC.
Figure 1. Atg37 regulates the assembly of the pexophagic RPC. Atg37 is an integral PMP, which, similar to the pexophagy receptor, Atg30, binds Pex3 in the peroxisomal membrane. Atg37 is also an acyl-CoA binding protein that binds either palmitoyl-CoA or Atg30. Atg30 recruits Atg37 to the pexophagic RPC where Atg37 facilitates the engagement of the scaffold protein Atg11. To bind both Atg11 and the phagophore protein Atg8, Atg30 has to be phosphorylated (P) at the corresponding binding sites. Acyl-CoA (AC) might regulate the Atg30-Atg37 interaction and as a consequence affect the recruitment of Atg11 to the pexophagic RPC.
In contrast to yeast, there is little known about the structure of the pexophagic RPC in mammalian cells. Despite the fact that human NBR1 can tag the peroxisomes for autophagic degradation, it is not a specific pexophagy receptor, since it can also recognize other membrane organelles and protein structures, such as late endosomes, midbodies, and ubiquitinated protein aggregates. We found that similar to yeast Atg37, human ACBD5/ATG37 is localized exclusively to the peroxisomes and is required specifically for pexophagy. It would be interesting to check, if ACBD5 interacts with NBR1 or its coreceptor, SQSTM1. If it does not, then ACBD5 could be used to affinity isolate a strictly pexophagy-specific mammalian receptor, the functional ortholog of the P. pastoris Atg30 and S. cerevisiae Atg36 proteins. In that case, determining the composition of the ACBD5-containing pexophagic RPC in mammalian cells would be the next breakthrough in the pexophagy field.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
I thank J C Farré for critical reading of the manuscript. This work was supported by the NIH grant, DK094843.
