Abstract
Our recent study revealed a new role of nucleus accumbens-1 (NAC1), a transcription factor belonging to the BTB/POZ gene family, in regulating autophagy. Moreover, we found that the high-mobility group box 1 (HMGB1), a chromatin-associated nuclear protein acting as an extracellular damage associated molecular pattern molecule (DAMP), is the downstream executor of NAC1 in modulating autophagy. In response to stress such as therapeutic insults, NAC1 increases the expression, cytosolic translocation and release of HMGB1; elevated level of the cytoplasmic HMGB1 leads to activation of autophagy. The NAC1-HMGB1 partnership may represent a previously unrecognized pathway that regulates autophagy in response to various stresses such as chemotherapy.
Autophagy is a tightly regulated catabolic activity that involves degradation of cellular components through the lysosomal machinery. It has now been increasingly appreciated that autophagy plays important roles in many physiological and pathophysiological processes such as development and growth, aging, innate immune response and cancer, helping maintain a balance between the synthesis, degradation, and subsequent recycling of cellular products. Understanding of the molecular mechanisms and pathways involved in the regulation of autophagy has also advanced greatly. Our recent finding that NAC1 partners with HMGB1 in modulating autophagy in response to therapeutic stress adds new members to the growing team of autophagy-regulatory components.
NAC1, a transcription factor belonging to the BTB/POZ gene family, is overexpressed in ovarian cancer and several other types of malignancies, and overexpression of NAC1 is correlative with tumor recurrence and resistance to chemotherapy. Yet, the precise mechanism of how NAC1 contributes to chemoresistance is largely unclear. We found that the functional status of NAC1 plays an essential role in the activation of cytoprotective autophagy in ovarian cancer cells treated with cisplatin, a commonly used chemotherapeutic drug in the treatment of ovarian cancer and other types of malignancies. We demonstrated that inactivation or silencing of NAC1 expression blunts the autophagic response triggered by cisplatin. Inactivation or silencing of NAC1 also significantly augments the cisplatin-induced cytotoxicity and apoptosis. Suppression of autophagy and activation of apoptosis by inhibiting NAC1 in the cisplatin-treated tumor cells suggest a regulatory role for NAC1 in the crosstalk between these two critical cellular processes.
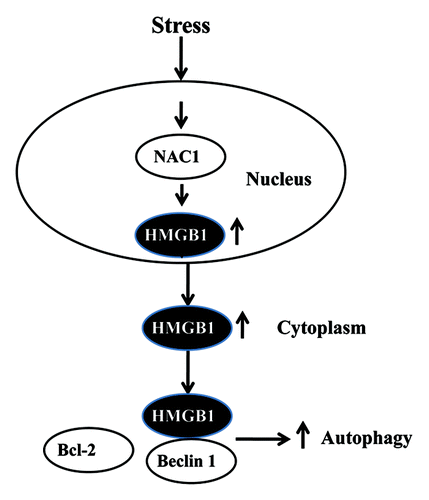
We further revealed a pathway by which NAC1 controls autophagy under stressful conditions, which is depicted in . We found that HMGB1 is a mediator of autophagy activated by NAC1. HMGB1, a chromatin-associated nuclear protein that also acts as an extracellular DAMP, is a Beclin 1-binding protein important in sustaining autophagy. Our study shows that HMGB1 is an essential downstream effector of NAC1-regulated autophagy, as knockdown of HMGB1 results in blunting of autophagy induced by cisplatin and other types of stress in cancer cells retaining active NAC1 function. Because HMGB1 modulates autophagy by disrupting the interaction between Beclin 1 and Bcl-2 in the cytoplasm, we next wanted to know how NAC1 collaborates with HMGB1 in controlling initiation of autophagy; or, more specifically, how NAC1 mobilizes HMGB1 to participate in regulating autophagy. As NAC1 predominantly localizes in the nuclei and acts as a transcription factor, we examined whether NAC1 activates the transcription of HMGB1, causing increases in the expression, translocation or release of HMGB1. Indeed, NAC1 appears to affect HMGB1 expression, as inactivation or silencing of NAC1 leads to a decrease in the expression of HMGB1 protein. Moreover, loss of function or knockdown of expression of NAC1 blocks the cytosolic translocation and release of HMGB1 in tumor cells treated with cisplatin, indicating that NAC1 also plays a role in boosting the movement and relocalization of HMGB1 under stress conditions. Thus, in response to stimuli such as cytotoxic insults, NAC1 activates autophagy by promoting the expression, translocation and release of HMGB1. The precise mechanism by which NAC1 controls the expression, translocation and release of HMGB1 remains to be elucidated. Also, whether NAC1 status affects the functions and activities of other autophagy-related molecules or pathways is another question that needs to be answered.
Figure 1. Regulation of autophagy by the NAC1-HMGB1 pathway. Under stress conditions, NAC1 increases the expression, cytosolic translocation, and release of HMGB1; in the cytoplasm, HMGB1 disrupts the binding of Beclin 1 with Bcl-2. Association of HMGB1 with Beclin 1 promotes autophagy activity.
The implication of NAC1-HMGB1-manipulated autophagy in cancer was evidenced in our study by its role in modulating tumor cell sensitivity to the cancer chemotherapeutic agent cisplatin. We observed that suppression of the NAC1-HMGB1-mediated autophagy results in enhanced cisplatin cytotoxicity and more apoptotic cell death, suggesting that induction of autophagy by NAC1 confers a survival advantage to tumor cells in the presence of cytotoxic drugs. Whether or not NAC1-regulated autophagy affects sensitivity of other chemotherapeutic drugs or other therapeutic stress, and what other roles it may play in cancer development and progression, are worth investigating.
In summary, our study identified a novel autophagy regulator, NAC1, and its teammate, HMGB1, in executing autophagy-regulatory function. We believe that these findings add a new paradigm to the regulatory networks of autophagy, and provide additional insight into how autophagy is activated in stressed cells.