Abstract
Autophagy is an innate immune defense against bacterial invasion. Recent studies show that two adaptor proteins, p62 and NDP52, are required for autophagy of the bacterial pathogen Salmonella enterica serovar Typhimurium (S. Typhimurium). However, it is not known why two different adaptors are required to target the same bacterial cargo to autophagy. Here we show that both adaptors are recruited to bacteria with similar kinetics, that they are recruited to bacteria independently of each other, and that depletion of either adaptor leads to impairment of antibacterial autophagy. Depletion of both adaptors does not synergistically impair autophagy, indicating they act in the same pathway. Remarkably, we observed that these adaptors do not colocalize, but rather form non-overlapping microdomains surrounding bacteria. We conclude that p62 and NDP52 act cooperatively to drive efficient antibacterial autophagy by targeting the protein complexes they coordinate to distinct microdomains associated with bacteria.
Results and Discussion
Macroautophagy (hereafter referred to as autophagy) is an important degradation pathway that targets long-lived proteins, damaged organelles and bacteria.Citation1,Citation2 Autophagy plays a key role in innate immunity by restricting bacterial replication in host cells. Previously, we showed that a population (∼30%) of S. typhimurium is targeted by autophagy during infection.Citation3 It appears that these bacteria are targeted by autophagy within Salmonella-containing vacuoles (SCV), since bacteria that colocalize with the autophagy marker LC3 also colocalize with markers of the SCV.Citation4 We found that bacterial replication is increased in autophagy-deficient cells.Citation3 In these cells, more bacteria are observed in the cytosol than control cells, suggesting autophagy acts to prevent bacterial escape from SCVs and/or to promote fusion with lysosomes.Citation3
We found that approximately 50% of LC3+ bacteria colocalize with ubiquitinated proteins (Ub+), suggesting that ubiquitin may serve as a signal to promote antibacterial autophagy.Citation3 In fact, two recent studies support this model.Citation4,Citation5 Randow and colleagues showed that NDP52 is recruited to Ub+ bacteria.Citation5 NDP52 is an adaptor protein that binds both ubiquitin and LC3, and also coordinates a signaling complex including Tank-binding kinase (TBK1), Sintbad and Nap1.Citation5 Our group showed that p62 (also known as SQSTM1) is recruited to Ub+ bacteria.Citation4 p62 can also bind both ubiquitin and LC3, and also coordinates a signal transduction complex.Citation6 Both groups showed that depletion of these individual adaptors results in impairment, but not complete suppression, of antibacterial autophagy.Citation4,Citation5 However, it remains unclear why depletion of individual adaptors can cause autophagy impairment. Indeed, these parallel studies prompt many questions. Do these adaptors act in the same pathway? Is their recruitment interdependent? Do they associate with each other in the same complexes? Owing to the general importance of these adaptors to autophagy, we decided to address these questions.
NDP52 and p62 are recruited to S. typhimurium with similar kinetics.
We examined adaptor recruitment to S. typhimurium. p62 and NDP52 were both recruited to bacteria, with maximal association seen at 60–90 min post infection (p.i.), the time when bacteria are targeted by autophagy, and declining sharply after 2 hours p.i. (). At 60 min p.i. ∼37% bacteria were positive for p62 and ∼32% bacteria were positive for NDP52 (). When examined at 60 min p.i., the vast majority of bacteria that were positive for one adaptor were also positive for the other; when selecting for p62+ bacteria, 94% were positive for NDP52 (). When selecting for NDP52+ bacteria, 91% were positive for p62 (). Therefore, p62 and NDP52 are recruited to the same bacteria, and with similar kinetics.
NDP52 and p62 can associate in a complex, but also localize to distinct compartments in the cell.
Given that both adaptors directly bind ubiquitin and LC3, we expected p62 and NDP52 to associate with each other. Indeed, we were able to co-immunoprecipitate p62-mCherry and NDP52-GFP from transfected HeLa cells with anti-GFP antibody (). As a control, we showed that anti-GFP antibody did not pull down p62-mCherry in cells co-expressing GFP. Rapamycin treatment and Salmonella-infection had little effect on the association of p62 and NDP52 when compared to untreated cells. We were also able to co-immunoprecipitate endogenous p62 with NDP52 from non-transfected cells (data not shown). Thus, our data indicate that p62 and NDP52 can associate in protein complexes. However, when we examined the subcellular distribution of these adaptors by immunofluorescence (endogenous levels, detected with antibodies), we observed puncta with one adaptor that did not colocalize with the other. As shown in , we could readily detect NDP52+ puncta in which p62 was absent (see inset) and p62+ puncta in which NDP52 was absent. Therefore, p62 and NDP52 can target distinct compartments in the cell.
NDP52 and p62 are both required for autophagy of Salmonella.
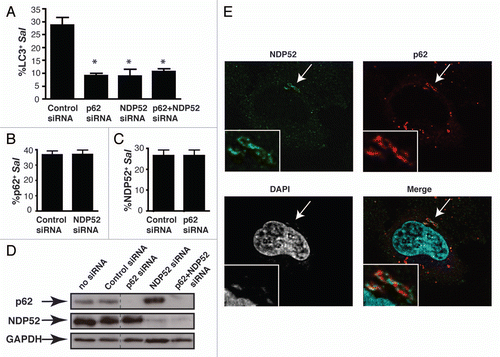
Previous studies have shown that knockdown of these adaptors results in impairment, but not complete suppression of antibacterial autophagy.Citation4,Citation5 To determine whether p62 and NDP52 function in the same pathway, we performed single and double knockdowns of adaptor proteins and measured the effect on autophagy by quantifying LC3+ intracellular bacteria. We observed that knockdown of either adaptor caused significant inhibition of LC3 recruitment to bacteria, confirming previous studiesCitation4,Citation5 (). However, double knockdown of both effectors (confirmed by western blotting in ) did not have an additive effect on autophagy impairment. Therefore, we conclude that p62 and NDP52 are not redundant and act in the same pathway to promote autophagy of S. typhimurium.
Adaptor proteins are recruited to the bacteria independently of one another.
To determine if adaptor recruitment is interdependent, we depleted expression of each adaptor singly, and examined its effect on the recruitment of the other adaptor to intracellular bacteria. Upon NDP52 depletion, p62 recruitment was not affected compared to control siRNA-treated cells (). Similarly, p62 depletion did not affect NDP52 recruitment to bacteria (). Since depletion of one adaptor did not affect the recruitment of the other, we conclude that p62 and NDP52 are recruited independently to bacteria.
NDP52 and p62 localize to non-overlapping microdomains around bacteria.
Upon examination of S. typhimurium positive for both adaptors, we were surprised to discover that they do not colocalize. Instead, we observed that p62 and NDP52 form independent non-overlapping micro-domains around the bacteria (). The microdomains were easily visible by confocal microscopy (see Suppl. Movie 1 and Suppl. Fig. 1), and often detectable by epifluorescence microscopy using a 100x objective (Leica). Adaptor microdomains often, though not always, encompassed the entire bacterium. We conclude that p62 and NDP52 target distinct microdomains associated with S. Typhimurirum, and therefore do not act in the same complex to promote autophagy of bacteria.
Materials and Methods
Bacteria strains, cell culture and pharmacological agents.
Wild-type S. typhimurium SL 1344 and bacteria expressing RFP were used for these studies. Infection was done as described previously in reference Citation4. HeLa human epithelial cells were obtained from the American Type Culture Collection. All cells were cultured in DMEM plus 10% FBS. Rapamycin treatment was previously described in reference Citation16.
Small interfering RNA (siRNA) treatment, plasmids and transfection.
p62 (no. M-010230-00) and siGenome nontargeting siRNA pool no. 2 (no. D-001206-14-20) were from Dharmacon. NDP52 (CALC°CO2), 5′-UUC AGU UGA AGC AGC UCU GUC UCC C-3′ were custom ordered from Sigma. siRNA knockdown and transfections were done as described in reference Citation4. Plasmids used in the study are GFP-LC3, and p62-mCherry that were previously described in reference Citation4, and NDP52-GFP (gift from J. Kendrick-Jones, University of Cambridge).Citation17
Immunofluorescence and confocal microscopy.
Cells were fixed and stained as previously described in reference Citation4. Mouse mAb to p62 was from BD Biosciences (610832); rabbit polyclonal Ab to NDP52 was from Abcam (ab68588). Rabbit polyclonal Ab to S. typhimurium (Salmonella O anti-serum group B factors 1, 4, 5 and 12) was from Becton Dickinson (240984). Samples were analyzed using a Zeiss Axiovert microscope (63x objective) and LSM 510 software. Confocal images were imported into Adobe Photoshop and assembled in Adobe Illustrator for labeling. Colocalization quantifications were performed using a Leica DMIRE2 epifluorescence microscope.
Immunoprecipitation and immunoblot.
The immunoprecipitation protocol was previously described in reference Citation18. Mouse anti-GFP (Invitrogen, A11120) was used for IP. Immunoblots were performed as described previously in reference Citation4. Blots were probed with rabbit anti-GFP (Invitrogen, A11122), mouse anti-p62 and mouse anti-GAPDH (Millipore, MAB374).
Conclusions
Our study provides novel insight into the mechanism by which ubiquitin-binding adaptor proteins, p62 and NDP52, target bacteria to the autophagy pathway. We demonstrate that these adaptors are both recruited to the same bacteria at the same time. However, p62 and NDP52 are recruited independently of one another. In addition, we show that p62 and NDP52 are not redundant and function in the same pathway, indicating that each adaptor brings unique components necessary to drive ubiquitin-dependent antibacterial autophagy. To our surprise, we found that the adaptors localize to distinct bacteria-associated microdomains.
How are these microdomains established? Differential ubiquitination events and their specific recognition by the adaptors may play a role. p62 preferentially binds K63-linked poly-ubiquitin chains, though it is also able to bind K48-linked chains and mono-ubiquitin.Citation7–Citation11 NDP52 binds monoubiquitin in vitro, but there are no studies examining NDP52 binding affinity to different types of ubiquitin.Citation5 It is possible that NDP52 does not bind poly-ubiquitin chains, and is recruited specifically to domains rich in monoubiquitin. It is noteworthy that the identity of ubiquitinated proteins associated with S. typhimurium is not known, nor are the E3 ligases that mediate these post-translational modifications during infection. Alternatively, p62 and NDP52 may be recruited to microdomains via other proteins that they associate with.
How do p62 and NDP52 contribute to autophagy? Through their binding to LC3, p62 and NDP52 may help recruit membrane to generate autophagosomes around bacteria. We found that bacteria targeted by autophagy are present in multilamellar structures, indicating the requirement of extensive membrane recruitment.Citation4 By targeting different microdomains associated with bacteria, p62 and NDP52 may mediate recruitment of membrane from different sources. This might be analogous to micropexophagy in yeast, where a specific membrane called the micropexophagy apparatus (MIPA) contributes to autophagy, in addition to membrane derived from the vacuole.Citation12 Alternatively, p62 and NDP52 may regulate autophagy through recruitment of the signaling complexes they coordinate.
Our studies highlight an area of growing concern in autophagy that deals with the requirement of multiple adaptors for Ub+-selective autophagy. p62 often acts in conjunction with other adaptors, including ALFY,Citation13 BAG3,Citation14 and NBR1,Citation15 though it has not been clear why both adaptors are required and why autophagy phenotypes are observed with depletion of either adaptor. Our data suggest that adaptor proteins may target microdomains on their cargo (protein aggregates, organelles, etc.,) and provide nonredundant signals or membrane recruitment to promote autophagy. This hypothesis, while exciting, requires further study.
Abbreviations
| Alfy | = | autophagy-linked FYVE protein |
| BAG3 | = | BAG family molecular chaperone regulator 3 |
| LC3 | = | microtubule-associated protein 1 light chain 3 |
| MIPA | = | micropexophagy apparatus |
| NDP52 | = | nuclear dot protein 52 kDa |
| Nod | = | nucleotide-oligomerization domain |
| SCV | = | Salmonella-containing vacuole |
| TBK1 | = | tank-binding kinase 1 |
| Ub | = | ubiquitin |
Figures and Tables
Figure 1 The ubiquitin-binding adaptors, p62 and NDP52, are recruited to S. typhimurium with the same kinetics. (A) HeLa cells were infected with S. typhimurium expressing RFP. Cells were fixed at the indicated time and stained with antibody to p62 or NDP52. The percentage of p62+ or NDP52+ bacteria was enumerated by fluorescence microscopy. At least 100 bacteria were counted for each time point. The experiment was conducted two times and error bars represent the range. (B) HeLa cells were infected for 1 h, fixed and stained for p62 and NDP52. The percentage of p62 colocalizing with NDP52+ bacteria (left part) and the percentage of NDP52 colocalizing with p62+bacteria (right part) were enumerated by fluorescence microscopy. At least 100 bacteria were counted for each condition. The average ± SD for three independent experiments is shown. (C) HeLa cells were transfected with p62-mCherry and ND P52-GFP or p62-mCherry and GFP. Then cells were infected with S. typhimurium (1 h), or treated with rapamycin (2 h), as indicated. HeLa lysates were immunoprecipitated (IP) with GFP antibody. Lysates and precipitates were then blotted (WB) for p62 and GFP. The lysate blot also contains the GFP alone band (27 kDa) which is not depicted in the figure. (D) HeLa cells were coimmunostained with p62 and NDP52 antibodies. Insets show higher magnification in the area indicated with an arrow.
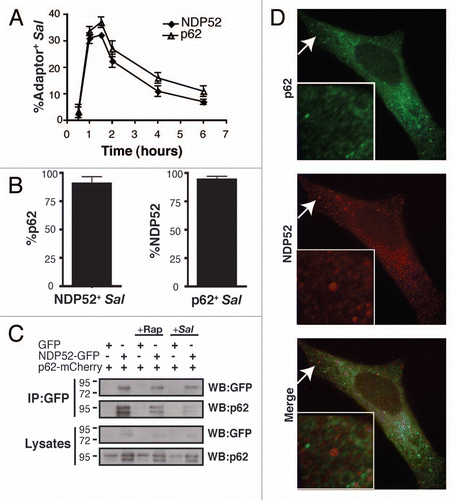
Figure 2 p62 and NDP52 are recruited independently to bacteria-associated microdomains to promote autophagy of S. typhimurium. (A) HeLa cells were treated with control, p62, NDP52 or p62 + NDP52 siRNA for 48 h and also transfected with GFP-LC3 for 24 h. Cells were then infected with S. typhimurium and fixed at 1 h p.i. Cells were then stained for external and internal bacteria. The percentage of LC3+ intracellular bacteria was enumerated by fluorescence microscopy. At least 100 bacteria were counted for each condition. The average ± SD is shown for three independent experiments. Asterisk denotes p value <0.001 calculated by two-tailed Student's t-test. (B and C) HeLa cells were transfected with the indicated siRN A and then infected with S. typhimurium for 1 h. The percentage of p62+ bacteria (B) and the percentage of NDP52+ bacteria (C) were enumerated by fluorescence microscopy. At least 100 bacteria were counted for each condition. The average ± SD for four independent experiments is shown. (D) Protein lysates from siRNA-treated cells were harvested and analyzed by western blot using antibodies to p62 and NDP52. GAPDH was used as a loading control. (E) HeLa cells were infected with S. typhimurium and fixed at 1 h p.i. Cells were then co-immunostained with p62 and NDP52 antibodies and stained with DAPI to label DNA. Insets show higher magnification in the boxed areas.
Additional material
Download Zip (2.1 MB)Acknowledgements
John H. Brumell, Ph.D., is the recipient of an Investigators in Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund. We thank Dr. John Kendrick-Jones (University of Cambridge, MRC) for kindly providing the NDP52-GFP construct, and to members of the Brumell laboratory for technical assistance and critical reading of this manuscript.
Addendum to:
References
- Huang J, Klionsky DJ. Autophagy and human disease. Cell Cycle 2007; 615:1837 - 1849
- Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science 2000; 2905497:1717 - 1721
- Birmingham CL, Smith AC, Bakowski MA, Yoshimori T, Brumell JH. Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole. J Biol Chem 2006; 28116:11374 - 11383
- Zheng YT, Shahnazari S, Brech A, Lamark T, Johansen T, Brumell JH. The adaptor protein p62/SQSTM1 targets invading bacteria to the autophagy pathway. J Immunol 2009; 1839:5909 - 5916
- Thurston TL, Ryzhakov G, Bloor S, von Muhlinen N, Randow F. The TBK1 adaptor and autophagy receptor NDP52 restricts the proliferation of ubiquitin-coated bacteria. Nat Immunol 2009; 1011:1215 - 1221
- Moscat J, Diaz-Meco MT. p62 at the crossroads of autophagy, apoptosis and cancer. Cell 2009; 1376:1001 - 1004
- Tan JM, Wong ES, Dawson VL, Dawson TM, Lim KL. Lysine 63-linked polyubiquitin potentially partners with p62 to promote the clearance of protein inclusions by autophagy. Autophagy 2007; 42
- Babu JR, Geetha T, Wooten MW. Sequestosome 1/p62 shuttles polyubiquitinated tau for proteasomal degradation. J Neurochem 2005; 941:192 - 203
- Okatsu K, Saisho K, Shimanuki M, Nakada K, Shitara H, Sou YS, et al. p62/SQSTM1 cooperates with Parkin for perinuclear clustering of depolarized mitochondria. Genes Cells 2010; 158:887 - 900
- Seibenhener ML, Babu JR, Geetha T, Wong HC, Krishna NR, Wooten MW. Sequestosome 1/p62 is a polyubiquitin chain binding protein involved in ubiquitin proteasome degradation. Mol Cell Biol 2004; 2418:8055 - 8068
- Wooten MW, Geetha T, Seibenhener ML, Babu JR, Diaz-Meco MT, Moscat J. The p62 scaffold regulates nerve growth factor-induced NFκB activation by influencing TRAF6 polyubiquitination. J Biol Chem 2005; 280:35625 - 35629
- Sakai Y, Oku M, van dK I, Kiel JA. Pexophagy: autophagic degradation of peroxisomes. Biochim Biophys Acta 2006; 176312:1767 - 1775
- Clausen TH, Lamark T, Isakson P, Finley K, Larsven KB, Brech A, et al. p62/SQSTM1 and ALFY interact to facilitate the formation of p62 bodies/ALIS and their degradation by autophagy. Autophagy 2010; 63:330 - 344
- Gamerdinger M, Hajieva P, Kaya AM, Wolfrum U, Hartl FU, Behl C. Protein quality control during aging involves recruitment of the macroautophagy pathway by BAG3. EMBO J 2009; 287:889 - 901
- Kirkin V, Lamark T, Johansen T, Dikic I. NBR1 cooperates with p62 in selective autophagy of ubiquitinated targets. Autophagy 2009; 55:732 - 733
- Shahnazari S, Yen WL, Birmingham CL, Shiu J, Namolovan A, Zheng YT, et al. A diacylglycerol-dependent signaling pathway contributes to regulation of antibacterial autophagy. Cell Host Microbe 2010; 82:137 - 146
- Morriswood B, Ryzhakov G, Puri C, Arden SD, Roberts R, Dendrou C, et al. T6BP and NDP52 are myosin VI binding partners with potential roles in cytokine signalling and cell adhesion. J Cell Sci 2007; 120:2574 - 2585
- Pankiv S, Johansen T. FYCO1: Linking autophagosomes to microtubule plus end-directing molecular motors. Autophagy 2010; 6:550 - 552