Abstract
Mitochondrial dysfunction has severe cellular consequences and is linked with neurodegenerative diseases and aging. Maintaining a healthy population of mitochondria is thus essential for proper cellular homeostasis. Several strategies have evolved to prevent and limit mitochondria damage, and macroautophagy plays a role in degrading superfluous or severely damaged mitochondria. Selective removal of mitochondria by autophagy (termed mitophagy) has been extensively studied recently in both yeast and mammalian cells. In this review, we summarize our current knowledge of mitophagy. We also compare the molecular process of mitophagy with other types of specific autophagic pathways and discuss its biological importance.
Introduction
Mitochondria are essential, dedicated organelles present in all eukaryotes.Citation1 Oxidative phosphorylation in mitochondria supplies energy for almost all cellular activities, while the accompanying production of reactive oxygen species (ROS) is potentially deleterious and may cause severe damage to mitochondrial proteins, DNA and lipids.Citation2,Citation3 Excessive ROS may eventually lead to cell death.Citation4 Accordingly, the amount and activity of mitochondria need to be tightly controlled to properly adapt the cell to the energy metabolic status, and it is also critical for cells to clear dysfunctional mitochondria in a timely manner. The primary mechanism cells use to eliminate mitochondria is macroautophagy (hereafter autophagy). Mitochondria were first detected inside an autophagosome in 1957.Citation5 Since then, autophagy has been hypothesized to play important roles in mitochondrial removal, and this is supported by recent studies in both yeasts and mammals.Citation6–Citation9 Selective autophagic mitochondria removal, mitophagy, is closely linked with normal quality control processes that prevent certain human pathophysiologies, especially neurodegenerative diseases such as Parkinson disease and Wolfram syndrome 2.Citation8,Citation10,Citation11 Recently, two yeast genome-wide screens designed to identify mitophagy-specific genes significantly broadened our understanding of mitophagy at the molecular level.Citation6,Citation7 Here, we review our current knowledge of the mechanism and biological significance of mitophagy, with a focus on the yeast model system.
Common Model for Selective Autophagy
Autophagy is an evolutionarily conserved cellular degradative process. In the last few years, the molecular mechanism and biological importance of autophagy have been extensively studied.Citation12 Our current understanding of this process has largely benefited from genetic screens done in yeast. To date, more than 30 autophagy-related (ATG) genes have been found to play important roles in autophagy. The morphological hallmark of autophagy is the formation of a double-membrane structure called an autophagosome.Citation13 Upon autophagy induction, a membrane structure termed the phagophore, the precursor to the autophagosome, gradually expands and engulfs a portion of the cytosol or specific cargos and delivers them to the vacuole/lysosome for degradation.Citation14 The diameter of a typical autophagosome is approximately 500 nm.Citation15 The mechanism of autophagosome formation, which entails the sequential expansion of the phagophore, provides autophagy with the capacity to sequester essentially any cellular component and deliver it into the vacuole for degradation. Indeed, autophagy plays a role in degrading a wide range of cellular components, such as protein complexes, the endoplasmic reticulum, peroxisomes, ribosomes and mitochondria.
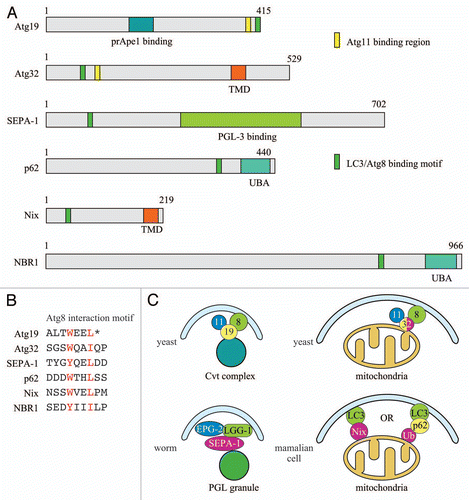
Although starvation-induced autophagy is thought to act nonselectively, autophagy can be highly selective when degrading a particular cargo, either a protein complex or an organelle, such as mitochondria.Citation16,Citation17 In yeast, several types of selective autophagy have been characterized including the cytoplasm to vacuole targeting (Cvt) pathway, pexophagy and mitophagy. These selective processes share some common features with regard to the molecular components. For example, all require the core autophagic machinery and other particular gene products for individual adaptation to different cargos. Among the 35 known ATG genes in yeast,Citation18 15 of the gene products are required for both nonspecific and specific autophagy, while others play particular roles in one or more types of autophagy. Atg19, Pichia pastoris Atg30 (PpAtg30) and Atg32 are the receptor proteins for the sequestration of the Cvt pathway cargo, peroxisomes and mitochondria, respectively.Citation6,Citation7,Citation19,Citation20 Atg19 is a soluble receptor that binds precursor aminopeptidase I to form the Cvt complex. PpAtg30 is a resident protein on peroxisomes, and Atg32 is a mitochondrial outer membrane protein. In the process of selective autophagy, these cargo receptor proteins are recognized by an adaptor protein Atg11, which is proposed to play a role in mediating cargo recognition and transport to the phagophore assembly site (PAS), the nucleating structure for generation of the phagophore.Citation21 Interaction of these receptor proteins with Atg8 may also play a role in cargo recognition. For example, Atg8 interacts with Atg19 and Atg32.Citation7,Citation22 The recognized cargo is subsequently enclosed within an autophagosome. The receptor-adaptor system likely represents a common mode of selective autophagy in yeast.
Another type of selective autophagy requires ubiquitination of the cargo or cargo subunits. For example, during autophagic degradation of ribosomes, ribophagy, ribosomal subunit Rpl25 is ubiquitinated, and this modification is required for ribophagy.Citation23 In this case, no receptor protein has been reported to be specifically required for the recognition of ribosomes. Although the Cvt pathway receptor, Atg19, is reported to be ubiquitinated,Citation24 the role of this ubiquitination needs more careful study. In mammalian cells, ubiquitination also plays an important role in cargo recognition.Citation25,Citation26 In this case, ubiquitin interacts with the Atg8 homologue LC3 via non-Atg adaptor proteins (for example, p62 and NBR1).Citation27,Citation28 Thus, ubiquitin may replace the role of the receptor. Accordingly, it will be interesting to see whether Atg11, which is a common adaptor for the receptors involved in pexophagy, mitophagy and the Cvt pathway, is required for ribophagy in yeast.
In C. elegans, the autophagic degradation of PGL granules is similar to the receptor-adaptor system in yeast. The PGL granule is marked by SEPA-1, and is then recognized by the protein EPG-2, which brings the PGL granule to the autophagic machinery through interaction with LGG-1/Atg8.Citation29,Citation30 SEPA-1 belongs to a protein family, and it will be interesting to see whether other SEPA-1 family proteins function as receptor proteins for the degradation of other cellular components in worms. In addition, it is not known whether EPG-2 is the functional equivalent of Atg11; this could be examined by determining whether EPG-2 is required for the other types of selective autophagy that are yet to be characterized in worms.
In summary, although the degradation of different cargo in different organisms may vary in their detailed mechanisms, it all requires one or more proteins (Atg11, Atg19, p62, EPG-2) that can specifically link the cargo to the autophagic machinery (). The specificity is provided by either a cargo resident protein (the aminopeptidase I propeptide, PpAtg30, Atg32, SEPA-1, Nix) or by modification/ubiquitination of the cargo (Rpl25, Vdac1, mitofusin1/2).
Atg32—Knowns and Many More Unknowns
Two independent genome-wide yeast screens identified Atg32 as the mitochondria receptor protein for mitophagy.Citation6,Citation7 Atg32 is not required for nonspecific autophagy or other kinds of selective autophagy, but only for mitophagy.Citation6,Citation7 As stated above, Atg32 interacts with Atg11 and Atg8, and these interactions are critical for mitochondria recruitment to the vacuole. Similar to Atg19, Atg32 has the W/YXXL/I motif that interacts with Atg8. The Cvt pathway is a biosynthetic pathway that constitutively delivers aminopeptidase I into the vacuole, whereas mitophagy is a tightly controlled process. Regulation of Atg32 may include important aspects that are involved in controlling mitophagy activity. For example, the interaction between Atg32 and Atg11 is significantly enhanced under mitophagy-inducing starvation conditions. Thus, it will be important to ascertain how this interaction is triggered and regulated. It is also not known whether this interaction also increases during post-log phase growth. Although the Atg32 protein level is not significantly changed during starvation conditions, it is reported that the level increases in the post-log phase during respiratory growth,Citation7 but how the Atg32 protein level is regulated requires further investigation. Mitophagy has been hypothesized to play a role in eliminating damaged mitochondria as part of the quality control mechanism that regulates the cellular mitochondria population. Therefore, it will be interesting to determine whether Atg32-mediated mitophagy has any preference for damaged versus functional mitochondria. Atg32 has an intramitochondrial domain at its C terminus. This could represent a potential module that can sense the status of mitochondria for the regulation of mitophagy.
The mammalian homologue or functional counterpart of Atg32 is yet to be discovered, although one recent study suggests that Nix might be the “mammalian Atg32.”Citation31 Nix is a mitochondrial outer membrane protein with the ability to bind to LC3/GABARAP.Citation32,Citation33 While Nix was initially reported to function in the maturation of reticulocytes,Citation34,Citation35 it was recently shown to be important also for CCCP-induced mitophagy of depolarized mitochondria.Citation32,Citation36 Other reports suggest that the recognition of mitochondria by autophagy may be achieved by p62 binding to mitochondria proteins such as Vdac1, mitofusin1 and mitofusin2 that have been ubiquitinated in a Parkin-mediated process.Citation25,Citation26 The relationship between Nix and Parkin requires further investigation.
Evolutionarily, mitochondria evolved as specialized organelles after the symbiotic engulfment of aerobic alpha-proteobacteria by pre-eukaryotic cells more than 1.5 billion years ago. It is intriguing to speculate that mitophagy may have played a role in the evolution of mitochondria in their transition from bacteria to organelles, potentially by allowing the degradation of parts of the proteobacteria that were redundant or otherwise incompatible with the pre-eukaryotic cells. Xenophagy, the selective autophagic degradation of cytosolic microbes, in some cases requires ubiquitination of the cytosolic bacteria.Citation37–Citation39 This process might also reflect the very early days of eukaryotic cell development. Different specialized mitochondria receptor proteins for mitophagy, such as Atg32 and Nix, may have been developed later in different species.
Mitophagy Induction and Regulation
In yeast, two major pathways that can induce mitophagy are starvation and post-log phase culture in a nonfermentable carbon source.Citation6,Citation7,Citation16 An ATG32 deletion mutant can survive starvation, however, as well as a wild-type strain.Citation6 Thus, the primary function of mitochondria removal during starvation does not appear to be for nutrient recycling. Therefore, the biological significance of starvation-induced mitophagy requires further investigation.
Mitophagy has also been well documented during post-log phase growth in a nonfermentable carbon source.Citation16,Citation40 It has been speculated that mitochondria elimination during post-log phase occurs to adapt cells to a reduced energy requirement.Citation18 An additional observation is that treatment with the ROS scavenger N-acetyl cysteine reduces mitophagy activity.Citation7 Thus, ROS production during the post-log phase under respiratory conditions may contribute to the upstream signaling for mitophagy.
Mitophagy is also induced in some mitochondria-related mutants. For example, defects in FoF1-ATPase biogenesis by the temperature sensitive mutant fmc1 or osmotic swelling of mitochondria by the depletion of the mitochondrial K+/H+ exchanger Mdm38 induce mitophagy.Citation41,Citation42 In both examples, a decrease in mitochondria membrane potential is observed. In mammalian cells, disruption of mitochondrial membrane potential by CCCP induces Parkin recruitment to the impaired mitochondria and subsequent removal by autophagy.Citation8 CCCP treatment, however, does not induce mitophagy in wild-type yeast. Thus, the relationship between mitochondrial membrane depolarization and mitophagy induction in yeast requires more study.
Future Directions
Despite recent advances in our knowledge of mitophagy, further questions remain to be addressed including: (1) What are the upstream signaling pathways that induce and regulate mitophagy? Although the molecular process of mitophagy has been characterized to some extent, we barely know the details of how mitophagy is initiated and how mitophagy activity is controlled. Recent research reports that the Bck1 signaling pathway is required for pexophagy;Citation43 Bck1 is also important for mitophagy.Citation44 Characterization of how this MAPK signaling pathway controls mitophagy will provide insights into mitophagy regulation. (2) What is the physiological role of mitophagy in yeast? As stated above, Atg32-depleted yeast strains display normal growth on a nonfermentable carbon source and also show no other apparent defects related to mitochondria morphology or function.Citation6,Citation7 How yeast cells adapt to the failure of mitochondrial removal is still a mystery, but perhaps other mitochondrial quality control pathways and cellular degradative pathways may compensate for the mitophagy defect. (3) Besides Atg32, more than 30 other genes are required for mitophagy based on two genome-wide mitophagy screens.Citation7,Citation44 Several of these genes previously were not known to be related with autophagic pathways, and the function of some of the gene products is not known. Figuring out how these genes are involved in mitophagy will further our understanding of this important cellular process.
Abbreviations
| Atg | = | autophagy-related |
| Cvt | = | cytoplasm to vacuole targeting |
| PAS | = | phagophore assembly site |
Figures and Tables
Figure 1 Selective autophagy and the corresponding organelle marker, receptor and adaptor proteins. The Atg11 interaction domain of Atg32 has not been mapped at present and the indicated position is approximate. (A) Key motifs of the marker and receptor proteins; (B) Atg8/LC3 interaction motifs of autophagic marker and receptor proteins; (C) Models of selective autophagy in different organisms. Atg32 shares properties of both organelle/cargo marker proteins and receptors; it is an integral membrane component of the mitochondria, and it binds both Atg11 and Atg8. TMD, transmembrane domain; UBA, ubiquitin-associated domain.
References
- Tatsuta T, Langer T. Quality control of mitochondria: protection against neurodegeneration and ageing. EMBO J 2008; 27:306 - 314
- McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol 2006; 16:551 - 560
- Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging and cancer: a dawn for evolutionary medicine. Annu Rev Genet 2005; 39:359 - 407
- Yen W-L, Klionsky DJ. How to live long and prosper: autophagy, mitochondria and aging. Physiology 2008; 23:248 - 262
- Clark SL Jr. Cellular differentiation in the kidneys of newborn mice studies with the electron microscope. J Biophys Biochem Cytol 1957; 3:349 - 362
- Kanki T, Wang K, Cao Y, Baba M, Klionsky DJ. Atg32 is a mitochondrial protein that confers selectivity during mitophagy. Dev Cell 2009; 17:98 - 109
- Okamoto K, Kondo-Okamoto N, Ohsumi Y. Mitochondria-anchored receptor Atg32 mediates degradation of mitochondria via selective autophagy. Dev Cell 2009; 17:87 - 97
- Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 2008; 183:795 - 803
- Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, et al. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol 2010; 8:e1000298
- Grunewald A, Voges L, Rakovic A, Kasten M, Vandebona H, Hemmelmann C, et al. Mutant Parkin impairs mitochondrial function and morphology in human fibroblasts. PLoS One 2010; 5:12962
- Geisler S, Holmstrom KM, Treis A, Skujat D, Weber SS, Fiesel FC, et al. The PINK1/Parkin-mediated mitophagy is compromised by PD-associated mutations. Autophagy 2010; 6:871 - 878
- Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol 2007; 8:931 - 937
- Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol 2007; 9:1102 - 1109
- Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ 2005; 12:1542 - 1552
- Xie Z, Nair U, Klionsky DJ. Atg8 controls phagophore expansion during autophagosome formation. Mol Biol Cell 2008; 19:3290 - 3298
- Kanki T, Klionsky DJ. Mitophagy in yeast occurs through a selective mechanism. J Biol Chem 2008; 283:32386 - 32393
- Kim J, Scott SV, Oda MN, Klionsky DJ. Transport of a large oligomeric protein by the cytoplasm to vacuole protein targeting pathway. J Cell Biol 1997; 137:609 - 618
- Kanki T, Klionsky DJ. The molecular mechanism of mitochondria autophagy in yeast. Mol Microbiol 2010; 75:795 - 800
- Scott SV, Guan J, Hutchins MU, Kim J, Klionsky DJ. Cvt19 is a receptor for the cytoplasm-to-vacuole targeting pathway. Mol Cell 2001; 7:1131 - 1141
- Farre JC, Manjithaya R, Mathewson RD, Subramani S. PpAtg30 tags peroxisomes for turnover by selective autophagy. Dev Cell 2008; 14:365 - 376
- Yorimitsu T, Klionsky DJ. Atg11 links cargo to the vesicle-forming machinery in the cytoplasm to vacuole targeting pathway. Mol Biol Cell 2005; 16:1593 - 1605
- Shintani T, Huang W-P, Stromhaug PE, Klionsky DJ. Mechanism of cargo selection in the cytoplasm to vacuole targeting pathway. Dev Cell 2002; 3:825 - 837
- Kraft C, Deplazes A, Sohrmann M, Peter M. Mature ribosomes are selectively degraded upon starvation by an autophagy pathway requiring the Ubp3p/Bre5p ubiquitin protease. Nat Cell Biol 2008; 10:602 - 610
- Baxter BK, Abeliovich H, Zhang X, Stirling AG, Burlingame AL, Goldfarb DS. Atg19p ubiquitination and the cytoplasm to vacuole trafficking pathway in yeast. J Biol Chem 2005; 280:39067 - 39076
- Geisler S, Holmstrom KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, et al. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol 2010; 12:119 - 131
- Gegg ME, Cooper JM, Chau KY, Rojo M, Schapira AH, Taanman JW. Mitofusin 1 and mitofusin 2 are ubiquitinated in a PINK1/parkin-dependent manner upon induction of mitophagy. Hum Mol Genet 2010; In press
- Kirkin V, Lamark T, Sou YS, Bjørkøy G, Nunn JL, Bruun JA, et al. A role for NBR1 in autophagosomal degradation of ubiquitinated substrates. Mol Cell 2009; 33:505 - 516
- Bjørkøy G, Lamark T, Brech A, Outzen H, Perander M, Øvervatn A, et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol 2005; 171:603 - 614
- Zhang Y, Yan L, Zhou Z, Yang P, Tian E, Zhang K, et al. SEPA-1 mediates the specific recognition and degradation of P granule components by autophagy in C. elegans. Cell 2009; 136:308 - 321
- Tian Y, Li Z, Hu W, Ren H, Tian E, Zhao Y, et al. C. elegans screen identifies autophagy genes specific to multicellular organisms. Cell 2010; 141:1042 - 1055
- Kanki T. Nix, a receptor protein for mitophagy in mammals. Autophagy 2010; 6:433 - 435
- Novak I, Kirkin V, McEwan DG, Zhang J, Wild P, Rozenknop A, et al. Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep 2010; 11:45 - 51
- Schwarten M, Mohrluder J, Ma P, Stoldt M, Thielmann Y, Stangler T, et al. Nix directly binds to GABARAP: a possible crosstalk between apoptosis and autophagy. Autophagy 2009; 5:690 - 698
- Sandoval H, Thiagarajan P, Dasgupta SK, Schumacher A, Prchal JT, Chen M, et al. Essential role for Nix in autophagic maturation of erythroid cells. Nature 2008; 454:232 - 235
- Schweers RL, Zhang J, Randall MS, Loyd MR, Li W, Dorsey FC, et al. NIX is required for programmed mitochondrial clearance during reticulocyte maturation. Proc Natl Acad Sci USA 2007; 104:19500 - 19505
- Ding WX, Ni HM, Li M, Liao Y, Chen X, Stolz DB, et al. Nix is critical to two distinct phases of mitophagy, reactive oxygen species-mediated autophagy induction and Parkin-ubiquitin-p62-mediated mitochondrial priming. J Biol Chem 2010; 285:27879 - 27890
- von Muhlinen N, Thurston T, Ryzhakov G, Bloor S, Randow F. NDP52, a novel autophagy receptor for ubiquitin-decorated cytosolic bacteria. Autophagy 2010; 6:288 - 289
- Ivanov S, Roy CR. NDP52: the missing link between ubiquitinated bacteria and autophagy. Nat Immunol 2009; 10:1137 - 1139
- Thurston TL, Ryzhakov G, Bloor S, von Muhlinen N, Randow F. The TBK1 adaptor and autophagy receptor NDP52 restricts the proliferation of ubiquitin-coated bacteria. Nat Immunol 2009; 10:1215 - 1221
- Tal R, Winter G, Ecker N, Klionsky DJ, Abeliovich H. Aup1p, a yeast mitochondrial protein phosphatase homolog, is required for efficient stationary phase mitophagy and cell survival. J Biol Chem 2007; 282:5617 - 5624
- Nowikovsky K, Reipert S, Devenish RJ, Schweyen RJ. Mdm38 protein depletion causes loss of mitochondrial K+/H+ exchange activity, osmotic swelling and mitophagy. Cell Death Differ 2007; 14:1647 - 1656
- Priault M, Salin B, Schaeffer J, Vallette FM, di Rago JP, Martinou JC. Impairing the bioenergetic status and the biogenesis of mitochondria triggers mitophagy in yeast. Cell Death Differ 2005; 12:1613 - 1621
- Manjithaya R, Jain S, Farre JC, Subramani S. A yeast MAPK cascade regulates pexophagy but not other autophagy pathways. J Cell Biol 2010; 189:303 - 310
- Kanki T, Wang K, Klionsky DJ. A genomic screen for yeast mutants defective in mitophagy. Autophagy 2010; 6:278 - 280