Abstract
New strategies to control infection with Mycobacterium tuberculosis, the causative agent of
tuberculosis, are urgently required, particularly in areas where acquired immune
immunodeficiences are prevalent. In this report we have determined if modification of the
current tuberculosis vaccine, Mycobacterium bovis BCG, to constitutively express the
mycobacterial HspX latency antigen altered its protective effect against challenge with
virulent M. tuberculosis. Overexpression of M. tuberculosis HspX in BCG caused reduced
growth in aerated cultures compared to control BCG, but growth under limited oxygen
availability was not markedly altered. Upon infection of mice, BCG:HspX displayed tissue-
specific attenuation compared to control BCG, with reduced growth within the lung and liver
but not the spleen. Both BCG:HspX and control BCG protected mice against aerosol M.
tuberculosis challenge to a similar extent, however, immunodeficient mice infected with
BCG:HspX survived significantly longer than mice infected with the control BCG strain.
Therefore, altering the in vivo persistence of BCG by overexpression of HspX may be one
important step towards developing a new tuberculosis vaccine with an improved safety profile
and suitable protective efficacy against M. tuberculosis infection.
Introduction
Mycobacterium tuberculosis remains the leading bacterial cause of death in humans, with tuberculosis resulting in approximately 2 million deaths per year.Citation1 Despite intense efforts to control the spread of M. tuberculosis, the incidence of the diseases is not decreasing, with over 9 million new cases of tuberculosis occurring annually.Citation1 The current vaccine, Mycobacterium bovis bacille Calmette-Guérin (BCG), is the only available vaccine approved for human use, however its use has not proved sufficient to control the spread of tuberculosis. For this reason there is much interest in the development of new vaccines strategies to replace BCG, with a small number of candidate vaccines now in human trials.Citation2 An important consideration for any new vaccine to be used to treat tuberculosis would be safety in immuno-compromised individuals, considering that tuberculosis is extremely problematic in areas of high HIV prevalence.Citation3 Thus there is a need to design new tuberculosis vaccines that display an adequate safety profile.
The rational design of a new tuberculosis vaccine is dependent on an understanding of the interaction of M. tuberculosis and the host, and using this knowledge to develop effective control strategies. During infection of the host, M. tuberculosis enters a period of latency or dormancy, which is characterized as a non-replicating state of low metabolic activity. A number of proteins that are specifically expressed during this dormancy period have been identified in both in vivo and in vitro models of latency. Much work has focussed on members of the DosR regulon, which regulates the expression of a large number of dormancy-associated proteins.Citation4 As expression of the proteins is associated with latent infection, they are considered potential biomarkers for latent tuberculosis, or possible components of vaccines that could be used to treat dormant M. tuberculosis. By far the most characterized of these proteins is HspX, a 16-kDa crystallin-like heat shock protein.Citation5 The HspX gene is highly upregulated within macrophages,Citation6 conditions which mimic the intracellular environment such as hypoxiaCitation6,Citation7 and presence of NO donors,Citation8 and HspX transcripts are more prevalent at late stages of M. tuberculosis infection in mice.Citation9 HspX is strongly recognized by T cells isolated from M. tuberculosis-infected individuals, and responses are more pronounced in those classified to have latent M. tuberculosis infection.Citation10 Intriguingly, although both M. tuberculosis and BCG share a common DosR regulon and express a functional form of HspX, BCG-vacinees do not recognize the protein.Citation11,Citation12 This suggests that BCG vaccination may not induce adequate responses to immunogenic latency antigens such as HspX, which may in part contribute to the lack of effectiveness of the vaccine.
In this report we constructed a BCG strain that constitutively expresses the HspX protein, in order to confer on BCG the capacity to induced HspX-reactive T cells. We show that HspX overexpression results in early recognition of the protein by the immune response. BCG:HspX displayed reduced virulence compared to BCG alone in immunodeficient animals, however both strains displayed a similar level of protective efficacy against M. tuberculosis infection in immunocompetent mice.
Results
Overexpression of HspX attenuates BCG growth under high-oxygen tension.
In order to determine the effect of HspX overexpression on BCG growth and protective efficacy, the strain BCG:HspX was constructed, in which the M. tuberculosis HspX gene is expressed in BCG under the control of the strong Mycobacterium bovis Hsp60 promoter.Citation13 This resulted in a number of BCG:HspX clones that displayed high levels of HspX expression in log-phase bacteria grown under aeration, which was not seen in BCG alone grown under the same conditions (). Overexpression of HspX correlated with an increased lag in growth of BCG:HspX when grown with aeration (shaking cultures), while growth compared to BCG alone was not affected in limited aeration of cultures (standing cultures; ). When BCG and BCG:HspX were inoculated into mice, only splenocytes from BCG:HspX-infected animals displayed significant levels of HspX-specific T cells secreting IFNγ, despite the fact that both strains induced comparable levels of reactive T cells recognising culture filtrate proteins from M. tuberculosis (CFP; ). These data suggests that constitutive overexpression of HspX in BCG compromises growth in high-oxygen conditions, and promotes early recognition of HspX by the immune system when delivered to mice.
In vivo attenuation of BCG:HspX.
We next determined if constitutive expression of HspX in BCG altered the capacity of the vaccine to persist within the host. To do this, mice were infected with BCG or BCG:HspX and bacterial loads in organs monitored over time. In both the lung and liver, bacterial numbers steadily decreased over time, however at both sites BCG:HspX was cleared more rapidly from these organs ( and B). In the spleen identical over the timecourse examined (). These results suggest that overexpression of HspX results in attenuation of BCG persistence within the host, which is most apparent within the lungs and liver of infected animals.
In order to further define the influence of HspX expression on BCG phenotype, we examined the virulence of rBCG strains in lymphocyte-deficient RAG-1-/- mice. Mice vaccinated with BCG alone survived had a median survival time of 161 days (). In mice immunized with BCG:HspX the median survival time was extended to 249 days, which was significantly greater than mice infected with BCG alone. Therefore the constitutive expression of HspX by BCG reduced the virulence of the vaccine in immunodeficient mice.
Protection against virulent M. tuberculosis infection afforded by HspX.
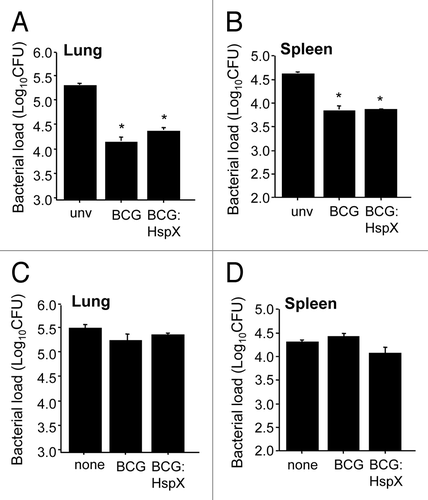
As the HspX antigen is recognized by individuals infected with M. tuberculosis and is highly expressed during the latent phase of infection,Citation9–Citation11 we determined if the high-level constitutive expression of HspX by BCG would alter the protective efficacy of the vaccine. To do this, mice were vaccinated with BCG or BCG:HspX, challenged at 12 weeks post-vaccination with aerosol M. tuberculosis, and bacteria loads in the organs determined. BCG:HspX reduced M. tuberculosis numbers in the lung and spleen of vaccinated mice compared to unvaccinated animals ( and B). BCG:HspX did not however significantly improve the protective effect afforded by BCG alone ( and B).
The use of latency antigens as post-exposure vaccines to target dormant bacteria is an area of considerable interest, as prophylactic vaccines may not be sufficient to induce sterilizing immunity.Citation14 As HspX is a latently expressed antigen we also assessed if BCG:HspX could improve clearance of M. tuberculosis post-infection. Delivery of either BCG or BCG:HspX at 8 weeks post M. tuberculosis-infection did not significantly reduce the bacterial burden compared to non-treated animals, and there was no difference in the effect observed between the two strains ( and D).
Discussion
The urgent need for new tuberculosis vaccines requires the development of new strategies to afford protective efficacy against this most difficult pathogen. We surmised that improving the capacity of the BCG vaccine to express the HspX latency antigen would allow early recognition of this antigen by the immune system, and potentially improve the protective effect of the vaccine. Overexpression of the protein increased the lag-phase of bacteria grown under conditions of aeration (). A similar effect is seen when HspX is overexpressed in M. smegmatis or M. tuberculosis,Citation15 and suggests that the protein may play a role in the shift to stationary phase, and high levels of HspX limits the ability of the bacteria to enter the phase of exponential growth. Intriguingly, when mice were infected with BCG:HspX, attenuation of growth was observed in the lung and liver but not the spleen. This may indicate that oxygen availability differs at these sites; indeed, granulomatous lesions in M. tuberculosis-infected murine lung do not appear hypoxic, which may limit induction of DosR-regulated genes and result in a delayed shift to latent infection.Citation16 Our results are also in agreement with the study of Hu et al. in which HspX deletion from M. tuberculosis results in increased growth within the mouse lung.Citation17
BCG vaccination does not result in recognition of HspX despite the fact that the HspX gene is intact and functional in BCG.Citation12 However, individuals infected with M. tuberculosis avidly recognize the protein. As BCG is presumably cleared relatively quickly from the vaccinated individual it is possible that the vaccine strains my not enter the ‘latent state’ of infection that is associated with M. tuberculosis infection and conducive to HspX expression.Citation9 Accordingly, we showed that early recognition of HspX by the immune response was only observed when BCG constitutively expresses HspX (). HspX is clearly immunogenic in this model, as the protein is recognized as a DNA vaccine in C57BL/6 mice (data not shown), with similar results observed in BALB/c mice.Citation18 This suggests that HpsX responses may occur late post-BCG vaccination, or antigen load may never reach the levels required for optimal stimulation of anti-HspX-immunity. This is an important consideration for the use of latently expressed antigens as vaccine components in humans.
Overexpression of HspX in BCG did not alter the protective efficacy of the vaccine against aerosol challenge, suggesting that the protein did not contribute to the protective antigenic repertoire of BCG (). We tested protective efficacy at an extended timepoint post-vaccination (8 weeks), on the basis that HspX would be more actively expressed by M. tuberculosis at later timepoints post challenge. It is possible however that greater protective effects are observed at more extended time- points, as significant HspX responses were detected at 9 months post-infection of mice with M. tuberculosis.Citation18 In addition, the coordinated overexpression of multiple dormancy antigens may be required to afford effective protective immunity, as a subset of these antigens are actively recognized by infected animals during latent M. tuberculosis infection.Citation18 This may also explain in part why overexpression of HspX did not improve the ability of BCG to reduce established M. tuberculosis infection in the lung (). As HspX is actively recognized by M. tuberculosis-infected individuals, it is also possible that protective responses may be more apparent in the human than observed in the mouse, and additional experiments in other relevant animal models of chronic M. tuberculosis infection may be required to further evaluate the potential of BCG:HspX as a M. tuberculosis vaccine. None-the-less, as BCG:HspX displayed protective efficacy similar to control BCG, yet was less virulent in immunodeficient mice (), the vaccine is a potential candidate to control tuberculosis in areas where high prevalence of immunodeficiencies may hamper the use of the current BCG vaccine.
Materials and Methods
Bacterial strains and media.
M. tuberculosis H37Rv (ATC27294) and M. bovis BCG Pasteur strain were grown in Middlebrook 7H9 medium (Difco Laboratories, Detroit, MI) supplemented with 0.05% Tween 80 and 10% ADC or on solid Middlebrook 7H11 medium (Difco Laboratories) supplemented with OADC. Bacteria were grown in liquid media either as rolling cultures or left standing for the required time period. When required, the antibiotic kanamycin (Km) was added at a concentration of 25 µg/ml.
Construction of recombinant BCG strains.
The gene encoding M. tuberculosis HspX was amplified from M. tuberculosis genomic DNA and cloned into the BalI and HindIII sites of pMV261,Citation13 to yield BCG:HspX. BCG containing pMV261 alone was used as the control strain for all experiments (BCG). Vectors were transformed into BCG as previously described.Citation19 To detect overexpression of recombinant HspX, bacterial lysates were probed by immunoblotting using sera from HspX immunized mice.
Immunogenicity and persistence studies.
All experiments using animals were approved by the University of Sydney Animal Ethics Committee. To assess T cell recognition of HspX, C57BL/6 mice (n = 4) were immunized subcutaneously (s.c) with 5 × 105 CFU of BCG:HspX or BCG. At 14 days single cell suspensions of splenocytes were prepared in RPMI medium containing 10% (v/v) FCS, 10 mM HEPES buffer, 10 mM sodium bicarbonate, 2 mM L-glutamine and 50 µM 2-mercaptoethanol. Antigen-specific IFNγ-secreting cells re-stimulated with recombinant HspX (3 µg/ml) or M. tuberculosis culture filtrate protein (CFP, 5 µg/ml) were measured by ELISPOT as previously described.Citation20
Persistence of BCG strain in mouse organs was determined by infection of mice intravenously (i.v) with 1 × 106 CFU of BCG:HspX or BCG and bacterial loads determined by plating of organ homogentates onto 7H11 media supplemented with Km (25 µg/ml).
Protective efficacy.
For assessment of protective efficacy, C57BL/6 mice (n = 5) were immunized with 5 × 105 CFU of BCG strains and at 12 weeks post-vaccination mice were challenged with aerosol M. tuberculosis H37Rv using a Middlebrook airborne infection apparatus (Glas-Col, Terre Haute, IN) with an infective dose of approximately 100 viable bacilli per lung. Four weeks after the challenge the number of bacteria within the lung and spleen was enumerated on Middlebrook 7H11 Bacto agar. Mice were also aerosol infected with M. tuberculosis as above and 8 week post vaccination were immunized s.c with 5 × 105 CFU BCG:HspX or BCG alone. Eight weeks post immunization the number of viable M. tuberculosis in the lung and spleen was determined.
For survival studies, RAG-1-/- mice (n = 4) were infected i.v. with 1 × 106 CFU of BCG:HspX or BCG. Infected mice were monitored daily and culled if displaying signs of ill health including reduced activity, ruffling of fur and weight loss (exceeding 15% of age-matched controls).
Statistical analysis.
The significance of differences for linear and log-transformed assays was evaluated by one-way ANOVA with pair-wise comparison of multi-grouped data sets achieved using the Bonferroni post hoc test. Survival was calculated using a Kaplan-Meier nonparametric survival plot, and significance was assessed by the Mantel-Cox log rank test.
Abbreviations
| BCG | = | bacille Calmette-Guérin |
| HspX | = | heat shock protein X |
| HIV | = | human immunodeficiency virus |
| ADC | = | albumin-dextrose-catalase |
| CFP | = | culture filtrate protein |
Figures and Tables
Figure 1 Overexpression of M. tuberculosis HspX in BCG. (A) The expression of HspX in rBCG strains was detected by immunoblotting using sera from HspX-immunized mice. 1, Markers; 2, BCG control; 3–5, BCG:HspX clones 1–3. (B) Growth in 7H9 media of BCG alone or BCG overexpressing HspX (BCG:HspX). Values represent mean OD600 nm of triplicate samples of cultures grown either under standing or shaking conditions. (C) Recognition of HspX in BCC immunized mice. Splenocytes from unvaccinated mice (unv) or mice immunized with BCG or BCG:HspX were cultured with 5 µg/ml CFP or 3 µg/ml HspX and the number of IFNγ-producing cells determined by ELISPOT at 14 days post-vaccination. The error bars represent standard errors of the means and data are representative of two separate experiments.
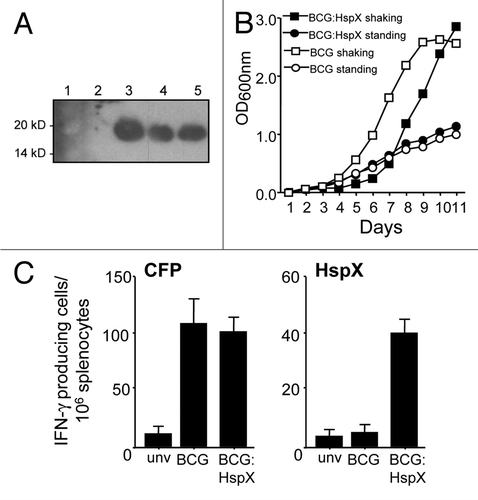
Figure 2 Persistence in mouse organs of BCG overexpressing HspX. Mice were however, number of both BCG and BCG:HspX were infected i.v with 1 × 106 CFU of BCG control (open squares) or BCG:HspX (closed circles). At 1, 14 and 28 and 56 days post-infection the bacterial load was assessed in the lung (A), liver (B) and spleen (C). The error bars represent standard errors of the means and data are representative of two separate experiments. The statistical significance between groups (*p < 0.05) was determined by ANOVA.
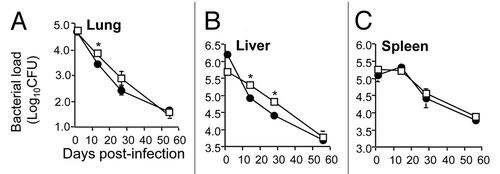
Figure 3 Safety of BCG:HspX in immuno-deficient mice. RAG-1-/- mice were infected i.v with 1 × 106 CFU of BCG control (open squares) or BCG:HspX (closed circles) and survival monitored over time. The significance of differences in survival was determined by Logrank-Mantel-Cox test (*p < 0.01). Data are representative of one of two individual experiments.
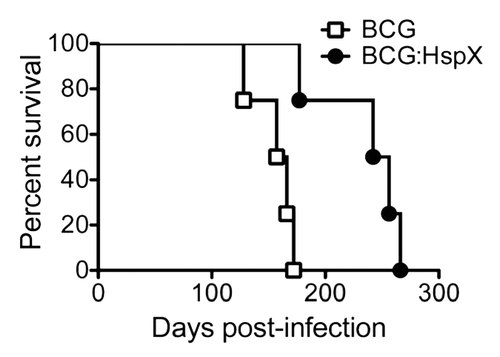
Figure 4 Protective efficacy of BCG overexpressing HspX. Mice were immunized s.c. with 5 × 105 CFU of BCG:HspX or BCG alone and 12 weeks post-immunization were challenged by aerosol route with M. tuberculosis H37Rv. Four weeks post-challenge the bacterial loads in the (A) lungs and (B) spleens were determined. Mice were also aerosol infected with M. tuberculosis as above and 8 week post vaccination were immunized s.c with 5 × 105 CFU BCG:HspX or BCG alone. Eight weeks post immunization the number of viable M. tuberculosis in the lung (C) and spleen (D) was determined. The statistical significance between naive and rBCG-vaccinated groups (*p < 0.01) were determined by ANOVA. Data are representative of two individual experiments.
Acknowledgements
This work was supported by the National Health and Medical Research Council of Australia. J. Triccas is the recipient of a NHMRC Biomedical Career Development Award. The HspX protein was received as part of NIH NIAID contract HHSN266200400091C awarded to Colorado State University.
References
- Dye C, Bassili A, Bierrenbach AL, Broekmans JF, Chadha VK, Glaziou P, et al. Measuring tuberculosis burden, trends and the impact of control programmes. Lancet Infect Dis 2008; 8:233 - 243
- Hoft DF. Tuberculosis vaccine development: goals, immunological design and evaluation. Lancet 2008; 372:164 - 175
- Vitoria M, Granich R, Gilks CF, Gunneberg C, Hosseini M, Were W, et al. The global fight against HIV/AIDS, tuberculosis and malaria: current status and future perspectives. Am J Clin Pathol 2009; 131:844 - 848
- Karakousis PC, Yoshimatsu T, Lamichhane G, Woolwine SC, Nuermberger EL, Grosset J. Dormancy phenotype displayed by extracellular Mycobacterium tuberculosis within artificial granulomas in mice. J Exp Med 2004; 200:647 - 657
- Chang Z, Primm TP, Jakana J, Lee IH, Serysheva I, Chiu W, et al. Mycobacterium tuberculosis 16-kDa antigen (Hsp16.3) functions as an oligomeric structure in vitro to suppress thermal aggregation. J Biol Chem 1996; 271:7218 - 7223
- Yuan Y, Crane DD, Simpson RM, Zhu YQ, Hickey MJ, Sherman DR, et al. The 16-kDa alpha-crystallin (Acr) protein of Mycobacterium tuberculosis is required for growth in macrophages. Proc Natl Acad Sci USA 1998; 95:9578 - 9583
- Sherman DR, Voskuil M, Schnappinger D, Liao R, Harrell MI, Schoolnik GK. Regulation of the Mycobacterium tuberculosis hypoxic response gene encoding alpha-crystallin. Proc Natl Acad Sci USA 2001; 98:7534 - 7539
- Garbe TR, Hibler NS, Deretic V. Response to reactive nitrogen intermediates in Mycobacterium tuberculosis: induction of the 16-kilodalton alpha-crystallin homolog by exposure to nitric oxide donors. Infect Immun 1999; 67:460 - 465
- Shi L, Jung YJ, Tyagi S, Gennaro ML, North RJ. Expression of Th1-mediated immunity in mouse lungs induces a Mycobacterium tuberculosis transcription pattern characteristic of nonreplicating persistence. Proc Natl Acad Sci USA 2003; 100:241 - 246
- Demissie A, Leyten EM, Abebe M, Wassie L, Aseffa A, Abate G, et al. Recognition of stage-specific mycobacterial antigens differentiates between acute and latent infections with Mycobacterium tuberculosis. Clin Vaccine Immunol 2006; 13:179 - 186
- Geluk A, Lin MY, van Meijgaarden KE, Leyten EM, Franken KL, Ottenhoff TH, et al. T-cell recognition of the HspX protein of Mycobacterium tuberculosis correlates with latent M. tuberculosis infection but not with M. bovis BCG vaccination. Infect Immun 2007; 75:2914 - 2921
- Lin MY, Geluk A, Smith SG, Stewart AL, Friggen AH, Franken KL, et al. Lack of immune responses to Mycobacterium tuberculosis DosR regulon proteins following Mycobacterium bovis BCG vaccination. Infect Immun 2007; 75:3523 - 3530
- Stover CK, de la Cruz VF, Fuerst TR, Burlein JE, Benson LA, Bennett LT, et al. New use of BCG for recombinant vaccines. Nature 1991; 351:456 - 460
- Andersen P. Vaccine strategies against latent tuberculosis infection. Trends Microbiol 2007; 15:7 - 13
- Yuan Y, Crane DD, Barry CE 3rd. Stationary phase-associated protein expression in Mycobacterium tuberculosis: function of the mycobacterial alpha-crystallin homolog. J Bacteriol 1996; 178:4484 - 4492
- Via LE, Lin PL, Ray SM, Carrillo J, Allen SS, Eum SY, et al. Tuberculous granulomas are hypoxic in guinea pigs, rabbits and nonhuman primates. Infect Immun 2008; 76:2333 - 2340
- Hu Y, Movahedzadeh F, Stoker NG, Coates AR. Deletion of the Mycobacterium tuberculosis alpha-crystallin-like hspX gene causes increased bacterial growth in vivo. Infect Immun 2006; 74:861 - 868
- Roupie V, Romano M, Zhang L, Korf H, Lin MY, Franken KL, et al. Immunogenicity of eight dormancy regulon-encoded proteins of Mycobacterium tuberculosis in DNA-vaccinated and tuberculosis-infected mice. Infect Immun 2007; 75:941 - 949
- Triccas J, Britton W, Gicquel B. Isolation of strong expression signals of Mycobacterium tuberculosis. Microbiology 2001; 147:1253 - 1258
- Palendira U, Kamath AT, Feng CG, Martin E, Chaplin PJ, Triccas JA, et al. Coexpression of interleukin-12 chains by a self-splicing vector increases the protective cellular immune response of DNA and Mycobacterium bovis BCG vaccines against Mycobacterium tuberculosis. Infect Immun 2002; 70:1949 - 1956