Abstract
Specific colonization of solid tumors by bacteria opens the way to novel approaches
in both tumor diagnosis and therapy. However, even non-pathogenic bacteria induce
responses by the immune system, which could be devastating for a tumor bearing
patient. As such effects are caused e.g. by the lipid A moiety of the
lipopolysaccharide, a msbB-mutant of the probiotic E. coli Nissle 1917 strain was
investigated. Bacteria of the mutant strain did not show any growth defects in culture
media when compared to wild-type E. coli Nissle 1917 but were unable to
myristoylate lipid A, had less toxic effects on immunocompetent BALB/c mice, and
were still able to specifically colonize tumors. Therefore, the modification of lipid A
could result in bacterial strains that might be better suited for diagnosis and therapy
of tumors than the corresponding wild-type strains, even if those are not considered
pathogenic or are of probiotic background.
Introduction
Despite the enormous amounts of research and clinical investigations, cancer remains a major health problem and causes millions of deaths each year. Although surgery is often successful, radio- and/or chemotherapy are widely used to cure malignant tumors and their metastases. However, severe side effects or relapse are often observed after these treatments.
Therefore, new strategies, which result in total regression of cancers and causing less side effects are desirable. One of these approaches is the development of specifically tumor colonizing, oncolytic bacterial strains, and in the last few years significant advances have already been achieved.Citation1–Citation17
Especially Clostridium spp. were regarded as potential weapons against cancer as these anaerobic bacteria can exclusively replicate in necrotic regions of solid tumors. In contrast to initial experiments which resulted in tumor regression but still caused the death of the animals as a consequence of the Clostridia, today genetically engineered bacterial species are used. These include virulence-attenuated mutants of pathogenic species that could be injected into mouse tumor models without causing disease, and/or expressing newly added heterologous therapeutic genes.Citation15
The use of Salmonella spp. has been particularly successful in many murine tumor modelsCitation2–Citation5,Citation7–Citation13,Citation18,Citation19 and the VNP20009 strain has even been tested in clinical trials in human cancer patientsCitation20,Citation21 and the maximum tolerated dose for intravenous injections was found to be 3 × 108 cfu/m2 in both trials.
The latter strain was, among other mutations which rendered the strain more tumor-specific, also depleted of the msbB (lpxM) gene which is responsible for myrstoylation of lipid A in many gram-negative bacteria.
Lipid A is a glucosamine-based lipid and serves as anchor of lipopolysaccharide (LPS) in the gram-negative outer membrane. It is also the portion of LPS which activates Toll-like receptor 4 (TLR4) and can lead to the syndrome of septic shock.Citation22–Citation24 The key deterrminants for the stimulation of TLR4 are the phosphate groups as well as the length and number of fatty acyl chains of lipid A.Citation25–Citation27 Therefore, variations and changes in the lipid A structure result in reduced TLR4 activation and it was shown that the described msbB mutants of Salmonella strain ATCC 14028 showed reduced toxicity to mice and swine but retained tumor colonization abilities.Citation28 For bacteria colonizing tumors for therapy the reduction in toxicity is a desirable characteristic to avoid side-effects. Therefore, a genetically stable variant VNP20009 which was defective for xyl, purI and msbB was generated and later on tested in clinical trials in human cancer patients.Citation20,Citation21,Citation29
Here, we constructed and analyzed a msbB-mutant of E. coli Nissle 1917, a strain which has successfully been used in tumor colonization studies.Citation17 In contrast to the previously mentioned Salmonella strains, this strain is not derived from pathogenic bacteria but was described to have probiotic properties. The strain E. coli Nissle 1917 does not encode any protein toxins nor mannose-resistant hemagglutinating adhesinsCitation30 but does still possess LPS-mediated endotoxin activity. A strain with non-myristoylated LPS should reduce the endotoxic effects and we therefore characterized and tested the ability to colonize tumors of a msbB-mutant E. coli Nissle 1917 strain to evaluate its potential in bacterial tumor therapy.
Results
Deletion of the msbB gene results in altered lipopolysaccharide composition of E. coli Nissle 1917.
To confirm the deletion of msbB in E. coli Nissle 1917 we performed PCR-analysis of the corresponding gene locus with lysates obtained from wild-type and msbB-mutant colonies of E. coli Nissle 1917. As expected the PCR results from colonies of the wild-type bacteria had a size of 1,282 bp while those of the deletion mutants were 963 bp smaller. Subsequent sequencing of the PCR products confirmed the replacement of the msbB gene by an EcoRI restriction site (data not shown).
More importantly, as the function of msbB is the myristoylation of the lipid A moiety of LPS,Citation31,Citation32 we analyzed the structure of the lipopolysaccharide in the wild-type and msbB-mutant strain of E. coli Nissle 1917. The deletion of msbB resulted in the formation of a non-myristoylated lipopolysaccharide as could be seen from the reduced size of the core structure (). Furthermore, the presence of pBWB536 resulted in a O6 side chain polymerization, which already was described for the wild-type strain of E. coli Nissle 1917.Citation33
In culture, deletion of msbB results in minor effects only.
The non-myristoylation of lipid A could have strong effects on gram-negative bacteria since LPS is a major part of the outer membrane in these bacteria. Previously, it was described that msbB mutants of Salmonella were unusually sensitive to certain growth conditions and polymixin B.Citation34 For E. coli such growth defects have not been observed, apart from increased deoxycholate resistance on solid agar assays in K-12 strainsCitation31,Citation32 and a clinical isolate of E. coli strain H16 (K-1 capsule) which formed filaments at 37°C upon msbB knock out. We therefore analyzed the effects of msbB deletion on the growth of E. coli Nissle 1917 (K-5 capsule) in LB medium supplemented with different compounds and also investigated whether the protein expression pattern of the bacteria was changed.
In the growth of wild-type E. coli Nissle 1917 and its msbB-mutant in LB medium was analyzed. No difference in replication rates were observed, indicating that the absence of myristoylation did not result in problems with nutrition uptake or other engery generating processes. In minimal inhibitory concentration (MIC) assays and on MSB-agar assays, we also found that the msbB-mutant compared to the wild-type strain does not show any major differences when grown in the presence of tetracycline, chloramphenicol, ampicillin, novobiocin, polymixin B or EGTA (data not shown). Furthermore, the formation of filaments could not be observed in either strain when growing at 37°C. However, we did find significant differences when analyzing the sensitivity to deoxycholate (). While almost no wild-type bacteria (0.5% recovery of plated bacteria) could grow on MSB-agar plates containing 2% deoxycholate at 30°C, about 80% of the msbB-mutant bacteria could form colonies under these conditions.
The expression of the outer membrane protein TolC was analyzed in more detail. This protein is a part of the multiprotein complex AcrB-AcrA-TolC, that confers resistance to a number of antibiotics.Citation35 But as expected from the results obtained with antibiotics like tetracycline, no significant differences were observed on the expression of this outer membrane protein (data not shown). We also analyzed the outer membrane protein expression pattern of the two strains. A few differences were observed on Coomassie strained SDS-gels (data not shown) but not investigated further as the primary goal was to characterize the behavior of the msbB-mutant strain in live mice and evaluate any reduced toxicity.
Induction of proinflammatory cytokines in cultures of J774 murine macrophages.
LPS triggers the activation of monocytes/macrophages leading to the secretion of proinflammatory molecules such as TNFα and IL-6.Citation36–Citation38 Together with the production of other cytokines and the activation of e.g., the coagulation cascade, fibrinolysis, production of lipid mediatiors and the complement pathway, multiple organ injury is induced during endotoxic shock.Citation36,Citation37 Therefore, we investigated whether the change in the lipid A structure of the msbB-mutant had any effects on the secretion of the proinflammatory molecules TNFα and IL-6 by murine macrophages.
Cells of the murine macrophage cell line J774 were co-incubated for 4 h with no bacteria, 2 × 104 and 2 × 106 cfu, respectively, of either wild-type or msbB-mutant E. coli Nissle 1917 bacteria. The concentration of TNFα and IL-6 was then determined and revealed significantly lower production of both cytokines when co-incubated with the msbB-mutant strain instead of the wild-type strain (). Non-myristoylation therefore resulted in less pronounced activation of murine macrophages, which should also result in reduced toxicity in live animals.
Decreased toxicity of msbB-mutants in BALB/c mice when compared to wild-type E. coli Nissle 1917.
The impact of msbB-deletion on the health of live-bacteria-injected BALB/C mice was investigated next. Therefore, the lethal dose killing 50% of the mice (LD50) was analyzed by intraperitoneal injection of different amounts of wild-type E. coli Nissle 1917 and its msbB-mutant respectively (). Each group of 4 mice received the different amounts of bacteria resuspended in 100 µl of PBS and survival was analyzed.
The data revealed a significant increase in survival upon administration of the msbB-mutant when compared to the wild-type E. coli Nissle 1917. The LD50 of the latter strain was in the range of 5 × 106 cfu, while about 10-fold more bacteria of the mutant strain were tolerated by BALB/c mice. Conclusively, the lipid A structure of the generated msbB-mutant strain of E. coli Nissle 1917 caused a less severe host response resulting in higher tolerance towards these bacteria.
Successful tumor colonization of both E. coli Nissle 1917 wild-type and msbB-deletion mutants.
The absence of myristoylation decreased the toxicity of the msbB-mutant strain, and so we wanted to investigate whether there were any effects upon the tumor-specific colonization as compared with the wild-type E. coli Nissle 1917.Citation17
Two days after injection of bacteria of the wild-type or the mutant strain, colony forming units of both strains were found in high concentration in tumor tissues with almost no background in spleen and liver (). The amount of msbB-mutant bacteria was lower compared to those of the wild-type strain, but still was detectable in very high concentrations.
We also tested whether the same bacterial strains equipped with higher serum-resistance behaved differently and could better colonize the tumor tissue due to prolonged half-life time in the serum. As can be seen from , those bacteria carrying the plasmid pBWB536, which encodes the genes necessary for synthesis of a full length O-antigen and resulting in a smooth phenotype of the bacteria,Citation33 did not have significantly better tumor colonizing abilities, nor were these strains present in spleen and liver in significantly higher concentrations. The serum resistance therefore has neither an impact on successful tumor colonization nor on specificity.
Discussion
Changes of LPS structure, especially of lipid A which can avoid the septic shock response are of clinical interest when considering the systemic injection of gram-negative bacteria into mammals.
Since many enterobacteria are able to specifically colonize solid tumors in live animalsCitation17 these bacteria might be well suited for tumor therapyCitation11,Citation39 and diagnosis.Citation40,Citation41 Consequently, myristoylation negative Salmonella strains derived from the Salmonella strain ATCC 14028 have been constructed, which were shown to have reduced toxicity in mice and swine but retained tumor-targeting in live animals 28. A particular strain (VNP20009) with suppressor mutations for msbB was tested in cancer patientsCitation20,Citation21,Citation29 and the maximum tolerated dose for intravenous injections was found to be 3 × 108 cfu/m2 in both trials.
Here, we analyzed a msbB-mutant of E. coli Nissle 1917. We think that this particular strain is especially useful for tumor therapy, as it was not derived from a pathogen but rather has probiotic activity. Furthermore, it does not express any protein toxins and even more importantly, the tumor to background ratio is much higher than that observed for Salmonella strains.Citation17 In contrast to the Salmonella strains that have already been used successfully in tumor therapeutic approaches, we were not yet able to observe tumor regression with the wild-type or the msbB-mutant E. coli Nissle 1917 strains. However, we think that controlled expression of defined toxins or even prodrug converting enzymes, e.g., through exogenous control by L-arabinose, L-rhamnose or anhydrotetracyclin administration,Citation17,Citation42 should lead to safe and well-tolerated bacterial strains which can be used in a therapeutic setting.
The deletion of msbB resulted in a strain which did not show any growth defects when compared to the wild-type E. coli Nissle 1917 strain growing in culture broth or in the presence of a number of antibiotics. This is similar to msbB-mutants of other E. coli strainsCitation31,Citation32 but different to Salmonella strains which usually grow slower compared to their isogeneic wild-type strains unless a suppressor mutation is inherited which confers this phenotype.Citation34,Citation43,Citation44 In line with previous observations made for msbB-mutants of other E. coli strains, the E. coli Nissle 1917 msbB-mutant also showed elevated resistance to the detergent deoxycholate when growing at 30°C on solid MSB-agar.Citation31,Citation32 It was discussed that the permeability barrier to deoxycholate may differ from that to other hydrophobic substances and that MsbB might affect this barrier,Citation31 which could also apply for E. coli Nissle 1917.
Interestingly, the concentration of msbB-mutant E. coli Nissle 1917 was lower in tumors 48 hpi when compared to the wild-type strain. Since no growth defects have been observed in culture the reason for this difference remains unexplained. It could be that the msbB-mutant strain either is growing slower in tumors of mice, or eliminated more effectively by the immune system of the mouse. The latter might indeed apply also for E. coli Nissle 1917, as a msbB-mutation in a clinical E. coli H16 isolate altered the cell surface interactions with complement and rendered the strain more susceptible to opsonization.Citation45 However, we did not observe the formation of filaments at 37°C, which was the case for the msbB-mutant strain of E. coli H16 and would have supported the assumption that the mutant strain is more prone to opsonization. Furthermore, when the E. coli Nissle 1917 msbB-mutant was equipped with an elongated O-antigen to prolong serum half-life using the pBWB536 plasmid,Citation33 no differences in terms of tumor colonization were observed.
Importantly, the msbB-mutant strain induced less pro inflammatory cytokines in murine macrophages than the corresponding wild-type strain. Consistent with that, the LD50 in BALB/c increased about 10-fold when the bacteria were injected intraperitoneally. Presumbly, this will also elevate the maximum tolerated dose in humans. As the successful colonization of solid tumors is also dependent on the amount of injected bacteria,Citation17 higher doses could lead to better tumor colonization rates and therefore optimized results in tumor diagnosis and therapy.
Materials and Methods
Bacterial strains and plasmids.
In this study we used a plasmid cured wild-type E. coli Nissle 1917 or a mutant thereof, which had a deletion of the msbB gene. If not otherwise indicated bacteria were grown in Luria Bertani (LB) medium. Bacteria containing the plasmid pBWB536 encoding for the wbO6 gene cluster of the uropathogenic E. coli strain 536,Citation33 were grown in LB supplemented with 100 µg/ml ampicillin.
Construction of the msbB mutant by homologous recombination.
The msbB locus of E. coli Nissle 1917 was sequenced after PCR amplification using primers MsbUPF and msbBR (for the sequence of primers see ) the amplicon was subjected to sequencing. On the basis of this sequence primers were designed to create a deletion of the msbB gene by overlap PCR using primers DmsbB1 (upstream forward), DmsbB2 (upstream reverse), DmsbB3 (downstream forward) and DmsbB4 (downstream reverse). The resultant fusion resulted in an amplicon flanked with SalI sites in which the complete msbB ORF was replaced with an EcoRI site (GAATTC). This was digested with SalI and ligated with pDM4 which had been similarly digested. The plasmid pDM4 encodes for sacB (lethal to E. coli grown on sucrose) and chloramphenicol resistance. Its replicon is dependent on the lambda PIR protein so this plasmid cannot replicate autonomously in E. coli Nissle 1917.Citation46 The ligation was used to transform E. coli PIR1 (Invitrogen, Carlsbad, CA, USA) and the trans formants were selected on LB agar containing chloramphenicol at 30 mg/l (Cm30). Transformants were subjected to miniprep and restriction analysis. A correct clone was chosen and the plasmid mobilised from E. coli PIR1 into E. coli Nissle 1917 by triparental mating, using E. coli HB101 with pRK2013 as helper strain.
Transconjugants were selected on MacConkey plates containing Cm30. On this medium Nissle shows faster growth and distinctive red colonies. Red colonies were picked and repeatedly subcultured on LB plates containing 5% sucrose to force the deletion of the sacB gene on the pDM4 plasmid backbone and thus select for double crossover events.
Resultant colonies were subjected to analysis by PCR across the msbB locus using the primers msbB-check-for and msbB-check-rev, sequencing of resultant amplicons and Southern blotting.
Analysis of the lipopolysaccharide expression.
Bacteria from 1 ml over night culture were harvested by centrifugation, re suspended in 100 µl Laemmli buffer, boiled for 10 min, and treated with Proteinase K (60°C, 60 min) to remove proteins. Samples were separated using a 20% polyacrylamid gel electrophoresis and analyzed by silver staining.
Minimal inhibitory concentration (MIC) of antibiotics and MSB-agar assays.
The MIC is defined as the lowest concentration of an antimicrobial agent that is sufficient to inhibit growth of bacterial cultures. For MIC-determination a serial dilution of antibiotics (ampicillin, chloramphenicol, ciprofloxacin, kanamycin, novobiocin, tetracyclin, polymyxin B) in LB medium was applied on a 96-well plate with 100 µl per well with antibiotic concetrations ranging from 0.02 to 500 µg/ml. Mid-logarithmic bacterial cultures were diluted (1:500) and 100 µl were added to each well. The plate was incubated over night at 37°C and then the optical density at 600 nm (OD600) was determined and plotted against the concentraion of the antibiotic. Cultures with OD600 values below 0.3 were defined as growth repressed. Typically cultures without antibiotic reached an OD600 of 0.7.
For MSB-agar assays bacteria were plated in serial dilutions on LB-0 or MSB-agar.Citation44 Sterile filtered (0.2 µm) stock solutions of deoxycholate (final concentration 0.25–2.0% w/v) or EGTA (final concentration 6.5 mM) (both substances from AppliChem GmbH, Darmstadt, Germany) were added after cooling of the agar to 45°C. Determination of relative survival occured by counting single colonies on MSB-agar without supplement and comparison to the number of colonies on MSB-agar plates containing deoxycholate.
Cytokine production.
For analysis of cytokine production of J774 murine macrophages upon contact with wild-type or msbB-mutant E. coli Nissle 1917 bacteria, cells were seeded on 12-well plates and incubated for 24 hours at 37°C in a 5% CO2-atmosphere. Bacterial overnight cultures were washed in endotoxin free PBS (PAA Laboratories, Pasching, Austria) and then dilutions were prepared in cell culture medium (Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum). The culture medium of the J774 cells was aspirated and replaced with bacteria containing medium or cell culture medium alone as negative control. After 4 h of co-incubation at 37°C in a 5% CO2-atmosphere, the medium was harvested and snap-frozen in liquid N2 before storage at −80°C until determination of TNFα and IL-6 production.
The concentration of TNFα and IL-6 was determined using the FlowCytomix™ Simplex Kits (Bender Medsystems GmbH, Vienna, Austria) according to the manufacturer's instructions. Samples were analyzed using a Epics XL flow cytometer equipped with a Low Angle Collection Kit (Beckman Coulter, Krefeld, Germany) and processed using FlowCytomixPro Version 2.2.1 software (Bender Medsystems GmbH).
Determination of LD50.
For the determination of the lethal dose resulting in the death of 50% of mice upon injection with bacteria of either E. coli Nissle 1917 wild-type or the msbB-deletion mutant strain, 50 ml LB medium were inoculated with 1 ml overnight culture of the respective strains. Bacteria were allowed to grow at 37°C and 180 rpm for about 90 minutes until the culture reached an OD600 of 0.4 corresponding to approximately 2 × 108 colony forming units (cfu) per millilitre LB medium.Citation17
Bacteria were then harvested by centrifugation, washed and diluted in PBS. Of these suspensions 100 µl were injected intraperitoneally into female six week old BALB/c mice and survival was analyzed 16 hours post injection.
Tumor colonization experiments.
Cells of the murine breast cancer cell line 4T1 (ATCC CRL-2539) were cultured in RPMI medium (PAA Laboratories, Pasching, Austria) supplemented with 10% fetal calf serum (PAA Laboratories). For induction of tumors, BALB/c mice were subcutaneously implanted on the right hind flank with 3.3 × 104 4T1 cells resuspended in 100 µl PBS. Two weeks after injection, tumors of about 1 cm diameter had formed. Bacterial inocula were prepared as already described for the determination of the LD50 but were administered intravenously into the tail vein. Forty-eight hours later, mice were sacrificed, tumors and organs were harvested and analyzed for the presence of bacteria. For this purpose, the tissues were homogenized and serial dilutions were plated on LB agar plates. The next day the number of colonies were counted and cfu/g tissue was calculated.
All animal experiments were carried out in accordance with protocols approved by the Institutional Animal Care and Use Committee (IACUC) of Explora Biolabs (San Diego, USA) or the ‘Regierung von Unterfranken’ (Würzburg, Germany).
Figures and Tables
Figure 1 Analysis of lipopolysaccharide structure in E. coli Nissle 1917 derived strains. The LPS of wild-type E. coli Nissle 1917 (EcN), msbB-mutant E. coli Nissle 1917 (ΔmsbB), or each strain containing the O-antigen encoding pBWB536 plasmid, was isolated and separated by gel electrophoresis. Subsequent silver gel staining revealed the presence of the core region and in case of pBWB536 containing bacteria, the O-antigen. The strains lacking the msbB gene had a reduced core size indicated by arrows.
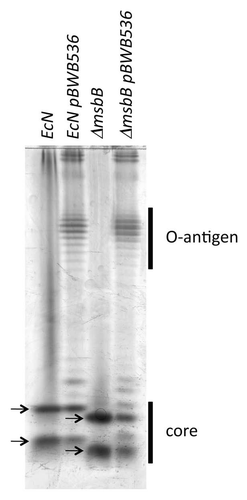
Figure 2 Growth of wild-type and msbB-mutant E. coli Nissle 1917. (A) E. coli Nissle 1917 wild-type (EcN) and msbB-mutant (αmsbB) were grown at 37°C in LB broth and no differences between the two strains were observed. (B) In contrast, growth of the wild-type bacteria on MSB-agar was inhibited by deoxycholate and only 0.5% of the plated cfu could be recovered while about 80% of the msbB-mutant bacteria formed colonies.
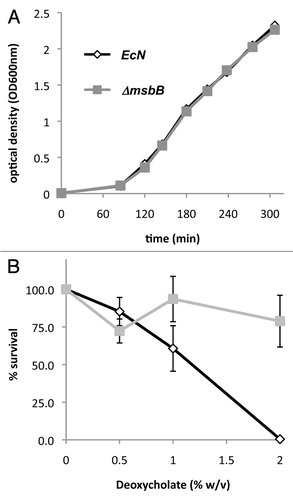
Figure 3 Induction of the proinflammatory cytokines TNFα and IL-6 in murine macrophages. Cells of the murine macrophage cell line J774 were co-incubated for 4 h with 2 × 104 or 2 × 106 cfu of wild-type (EcN, dark grey) and msbB-mutant (ΔmsbB, light grey) E. coli Nissle 1917, respectively. The formation and secretion of TNFα (A) and IL-6 (B) was then determined in the supernatant. Supernatants of cells treated with medium alone served as control and background levels (118 pg/ml for TNFα and 0 pg/ml for IL-6) were substracted from the obtained results. The data show significantly lower amounts of both proinflammatory cytokines when treated with the msbB-mutant strain.
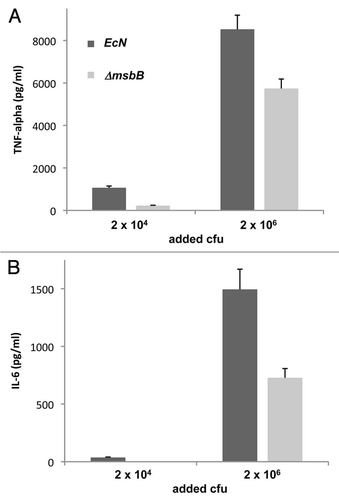
Figure 4 LD50 of E. coli Nissle 1917 strains in BALB/c mice. Different amounts of wild-type (EcN) and msbB-mutant (ΔmsbB) E. coli Nissle 1917 were i.p. injected into BALB/c mice. Latter bacterial strain resulted in reduced toxicity and therefore higher survival rates (about 10-fold higher LD50) when compared to the wild-type strain.
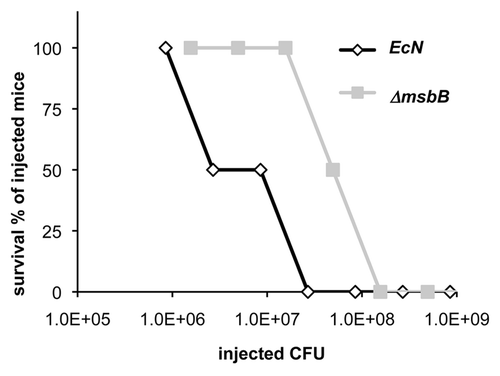
Figure 5 Tumor colonization of E. coli Nissle 1917 strains. BALB/c mice bearing 4T1 tumors were intravenously injected with 1 × 106 cfu of wild-type and msbB-mutant E. coli Nissle 1917, respectively. In addition, the bacteria of the same strains were injected which harbored the plasmid pBWB536. The plasmid leads to the synthesis of O-antigen conferring serum resistance. Two days post injection, tumor, liver and spleen of each mouse were isolated and analyzed for the presence of bacteria. Each strain was able to selectively colonize the tumor tissue with almost no background levels in spleen and liver.
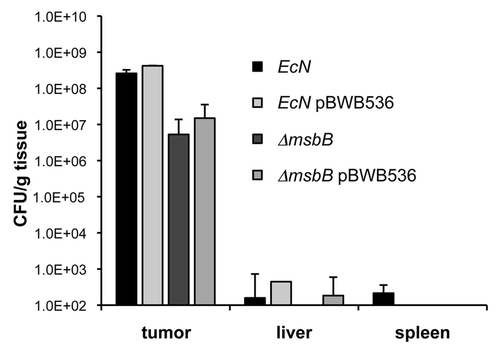
Table 1 Primers used in this study
Acknowledgements
The authors would like to thank Prof. Dr. U. Fischer for his support in general, Ms. J. Langbein for excellent technical assistance and Dr. T. Oelschlaeger for providing pBWB536 plasmid DNA. The work was supported by a research grant from Genelux Corp.
References
- Cheong I, Huang X, Bettegowda C, Diaz LA Jr, Kinzler KW, Zhou S, et al. A bacterial protein enhances the release and efficacy of liposomal cancer drugs. Science 2006; 314:1308 - 1311
- Fu W, Chu L, Han X, Liu X, Ren D. Synergistic antitumoral effects of human telomerase reverse transcriptase-mediated dual-apoptosis-related gene vector delivered by orally attenuated Salmonella enterica Serovar typhimurium in murine tumor models. J Gene Med 2008; 10:690 - 701
- Fu W, Lan H, Li S, Han X, Gao T, Ren D. Synergistic antitumor efficacy of suicide/ePNP gene and 6-methylpurine 2′-deoxyriboside via Salmonella against murine tumors. Cancer Gene Ther 2008; 15:474 - 484
- Friedlos F, Lehouritis P, Ogilvie L, Hedley D, Davies L, Bermudes D, et al. Attenuated Salmonella targets prodrug activating enzyme carboxypeptidase G2 to mouse melanoma and human breast and colon carcinomas for effective suicide gene therapy. Clin Cancer Res 2008; 14:4259 - 4266
- al-Ramadi BK, Fernandez-Cabezudo MJ, El-Hasasna H, Al-Salam S, Bashir G, Chouaib S. Potent anti-tumor activity of systemically-administered IL2-expressing Salmonella correlates with decreased angiogenesis and enhanced tumor apoptosis. Clin Immunol 2009; 130:89 - 97
- Ryan RM, Green J, Williams PJ, Tazzyman S, Hunt S, Harmey JH, et al. Bacterial delivery of a novel cytolysin to hypoxic areas of solid tumors. Gene Ther 2009; 16:329 - 339
- Hayashi K, Zhao M, Yamauchi K, Yamamoto N, Tsuchiya H, Tomita K, et al. Cancer metastasis directly eradicated by targeted therapy with a modified Salmonella typhimurium. J Cell Biochem 2009; 106:992 - 998
- Loeffler M, Le'Negrate G, Krajewska M, Reed JC. Salmonella typhimurium engineered to produce CCL21 inhibit tumor growth. Cancer Immunol Immunother 2009; 58:769 - 775
- Loeffler M, Le'Negrate G, Krajewska M, Reed JC. IL-18-producing Salmonella inhibit tumor growth. Cancer Gene Ther 2008; 15:787 - 794
- Loeffler M, Le'Negrate G, Krajewska M, Reed JC. Inhibition of tumor growth using salmonella expressing Fas ligand. J Natl Cancer Inst 2008; 100:1113 - 1116
- Mengesha A, Dubois L, Chiu RK, Paesmans K, Wouters BG, Lambin P, et al. Potential and limitations of bacterial-mediated cancer therapy. Front Biosci 2007; 12:3880 - 3891
- Loeffler M, Le'Negrate G, Krajewska M, Reed JC. Attenuated Salmonella engineered to produce human cytokine LIGHT inhibit tumor growth. Proc Natl Acad Sci USA 2007; 104:12879 - 12883
- Zhang L, Gao L, Zhao L, Guo B, Ji K, Tian Y, et al. Intratumoral delivery and suppression of prostate tumor growth by attenuated Salmonella enterica serovar typhimurium carrying plasmid-based small interfering RNAs. Cancer Res 2007; 67:5859 - 5864
- Royo JL, Becker PD, Camacho EM, Cebolla A, Link C, Santero E, et al. In vivo gene regulation in Salmonella spp. by a salicylate-dependent control circuit. Nat Methods 2007; 4:937 - 942
- Wei MQ, Ellem KA, Dunn P, West MJ, Bai CX, Vogelstein B. Facultative or obligate anaerobic bacteria have the potential for multimodality therapy of solid tumours. Eur J Cancer 2007; 43:490 - 496
- Yu YA, Shabahang S, Timiryasova TM, Zhang Q, Beltz R, Gentschev I, et al. Visualization of tumors and metastases in live animals with bacteria and vaccinia virus encoding light-emitting proteins. Nat Biotechnol 2004; 22:313 - 320
- Stritzker J, Weibel S, Hill PJ, Oelschlaeger TA, Goebel W, Szalay AA. Tumor-specific colonization, tissue distribution, and gene induction by probiotic Escherichia coli Nissle 1917 in live mice. Int J Med Microbiol 2007; 297:151 - 162
- Pawelek JM, Low KB, Bermudes D. Bacteria as tumour-targeting vectors. Lancet Oncol 2003; 4:548 - 556
- King I, Itterson M, Bermudes D. Tmor-targeted Salmonella typhimurium overexpressing cytosine deaminase: a novel, tumor-selective therapy. Methods Mol Biol 2009; 542:649 - 659
- Cunningham C, Nemunaitis J. A phase I trial of genetically modified Salmonella typhimurium expressing cytosine deaminase (TAPET-CD, VNP20029) administered by intratumoral injection in combination with 5-fluorocytosine for patients with advanced or metastatic cancer. Protocol no: CL-017. Version: April 9, 2001. Hum Gene Ther 2001; 12:1594 - 1596
- Toso JF, Gill VJ, Hwu P, Marincola FM, Restifo NP, Schwartzentruber DJ, et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J Clin Oncol 2002; 20:142 - 152
- Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature 2000; 406:782 - 787
- Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem 2002; 71:635 - 700
- Kotani S, Takada H, Tsujimoto M, Ogawa T, Takahashi I, Ikeda T, et al. Synthetic lipid A with endotoxic and related biological activities comparable to those of a natural lipid A from an Escherichia coli re-mutant. Infect Immun 1985; 49:225 - 237
- Golenbock DT, Hampton RY, Qureshi N, Takayama K, Raetz CR. Lipid A-like molecules that antagonize the effects of endotoxins on human monocytes. J Biol Chem 1991; 266:19490 - 19498
- Loppnow H, Brade H, Durrbaum I, Dinarello CA, Kusumoto S, Rietschel ET, et al. IL-1 induction-capacity of defined lipopolysaccharide partial structures. J Immunol 1989; 142:3229 - 3238
- Rietschel ET, Kirikae T, Schade FU, Mamat U, Schmidt G, Loppnow H, et al. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J 1994; 8:217 - 225
- Low KB, Ittensohn M, Le T, Platt J, Sodi S, Amoss M, et al. Lipid A mutant Salmonella with suppressed virulence and TNFα induction retain tumor-targeting in vivo. Nat Biotechnol 1999; 17:37 - 41
- Clairmont C, Lee KC, Pike J, Ittensohn M, Low KB, Pawelek J, et al. Biodistribution and genetic stability of the novel antitumor agent VNP20009, a genetically modified strain of Salmonella typhimurium. J Infect Dis 2000; 181:1996 - 2002
- Blum G, Marre R, Hacker J. Properties of Escherichia coli strains of serotype O6. Infection 1995; 23:234 - 236
- Karow M, Georgopoulos C. Isolation and characterization of the Escherichia coli msbB gene, a multicopy suppressor of null mutations in the high-temperature requirement gene htrB. J Bacteriol 1992; 174:702 - 710
- Somerville JE Jr, Cassiano L, Bainbridge B, Cunningham MD, Darveau RP. A novel Escherichia coli lipid A mutant that produces an anti-inflammatory lipopolysaccharide. J Clin Invest 1996; 97:359 - 365
- Grozdanov L, Zahringer U, Blum-Oehler G, Brade L, Henne A, Knirel YA, et al. A single nucleotide exchange in the wzy gene is responsible for the semirough O6 lipopolysaccharide phenotype and serum sensitivity of Escherichia coli strain Nissle 1917. J Bacteriol 2002; 184:5912 - 5925
- Murray SR, Bermudes D, de Felipe KS, Low KB. Extragenic suppressors of growth defects in msbB Salmonella. J Bacteriol 2001; 183:5554 - 5561
- Nikaido H. Multidrug resistance in bacteria. Annu Rev Biochem 2009; 78:119 - 146
- Lin WJ, Yeh WC. Implication of Toll-like receptor and tumor necrosis factor alpha signaling in septic shock. Shock 2005; 24:206 - 209
- Liang B, Gardner D, Griswold D, Song X-yR. Protection against Lipopolysaccharide-induced death by an anti-Interleukin-6 monoclonal Antibody. Am J Immunol 2007; 3:4 - 9
- Hack CE, De Groot ER, Felt-Bersma RJ, Nuijens JH, Strack Van Schijndel RJ, Eerenberg-Belmer AJ, et al. Increased plasma levels of interleukin-6 in sepsis. Blood 1989; 74:1704 - 1710
- Jia LJ, Hua ZC. Development of bacterial vectors for tumor-targeted gene therapy. Methods Mol Biol 2009; 542:131 - 154
- Soghomonyan SA, Doubrovin M, Pike J, Luo X, Ittensohn M, Runyan JD, et al. Positron emission tomography (PET) imaging of tumor-localized Salmonella expressing HSV1-TK. Cancer Gene Ther 2005; 12:101 - 108
- Brader P, Stritzker J, Riedl CC, Zanzonico P, Cai S, Burnazi EM, et al. Escherichia coli Nissle 1917 facilitates tumor detection by positron emission tomography and optical imaging. Clin Cancer Res 2008; 14:2295 - 2302
- Loessner H, Bartels S, Endmann A, Westphal-Daniel K, Wolf K, Kochruebe K, et al. Drug-inducible remote control of gene expression by probiotic Escherichia coli Nissle 1917 in intestine, tumor and gall bladder of mice. Microbes Infect 2009; 11:1097 - 1105
- Murray SR, de Felipe KS, Obuchowski PL, Pike J, Bermudes D, Low KB. Hot spot for a large deletion in the 18- to 19-centisome region confers a multiple phenotype in Salmonella enterica serovar typhimurium strain ATCC 14028. J Bacteriol 2004; 186:8516 - 8523
- Murray SR, Ernst RK, Bermudes D, Miller SI, Low KB. pmrA(Con) confers pmrHFIJKL-dependent EGTA and polymyxin resistance on msbB Salmonella by decorating lipid A with phosphoethanolamine. J Bacteriol 2007; 189:5161 - 5169
- Somerville JE Jr, Cassiano L, Darveau RP. Escherichia coli msbB gene as a virulence factor and a therapeutic target. Infect Immun 1999; 67:6583 - 6590
- Milton DL, O'Toole R, Horstedt P, Wolf-Watz H. Flagellin A is essential for the virulence of Vibrio anguillarum. J Bacteriol 1996; 178:1310 - 1319