Abstract
Mycobacterium avium subsp. paratuberculosis (MAP) is the etiological agent of Johne’s disease,
a granulomatous enteritis of cattle and other domesticated and wild ruminant species. Johne’s
disease is prevalent worldwide and has a significant impact on the global agricultural economy.
Current vaccines against Johne’s are insufficient in stemming its spread, and associated side-
effects prevent their widespread use in control programs. Effective and safe vaccine strategies
are needed. The main purpose of this paper is to propose and evaluate the development of a
novel oral subunit-vaccine using a patho-biotechnological approach. This novel strategy, which
harnesses patho-genetic elements from the intracellular pathogen Listeria monocytogenes, may
provide a realistic route towards developing an effective next generation subunit vaccine against
Johne’s disease and Paratuberculosis.
Introduction
Mycobacterium avium subspecies paratuberculosis (MAP) is a slow growing, Gram positive, acid fast, intracellular pathogenic Bacillus and the etiological agent of Johne disease in cattle. This is a chronic untreatable granulomatous enteritis with symptoms including intestinal inflammation, poor nutrient uptake, severe diarrhoea, emaciation and eventual death of the infected host.Citation1 Paratuberculosis infection has been demonstrated mainly in ruminants including cattle, sheep, rabbits, bison and red deer.Citation1–Citation3
Johne disease is prevalent in cattle worldwide and as such, has a significant impact on the global economy.Citation4 The U.S dairy industry alone has reported annual losses ranging from US$200 million to 1.5 billion due to infection.Citation4,Citation5 These costs are mainly attributed to decreased milk production, weight loss and increased culling measures. In Ireland, substantial economic losses have also occurred consequent to the presence of Johne disease within dairy herds.Citation6
MAP contaminates and persists in water and the environment, can survive milk pasteurisation and is present in dairy and meat products from infected animals.Citation7 It is therefore inevitable that human populations are widely exposed.Citation7 An overwhelming balance of probability and public health risk favours the conclusion that MAP is also pathogenic to humans.Citation7 It is a strong candidate pathogen in the development of Crohn disease, a human systemic disorder involving chronic inflammation of the intestine, similar to that observed in Johne disease infected animals.Citation7,Citation8
Vaccination Against MAP
There is currently no cure for Johne disease and hygienic measures or culling procedures are not sufficient to prevent its spread.Citation9 Moreover, the available vaccines against Johne disease do not fully protect animals from infection, but rather reduce clinical symptoms and limit shedding of MAP in feces.Citation9 While several whole cell vaccines have been developed, based on heat killed or live attenuated strains of MAP, issues related to their use prevents their widespread application in control strategies.Citation9,Citation10 The associated drawbacks with these vaccines include (1) the presence of local granulomatous lesions at the site of injection, (2) interference with current serodiagnostic tests for MAP and (3) failure to fully protect animals from subsequent exposure to MAP. Administration of vaccines developed from whole organisms also increases the likelihood of interference with bovine tuberculosis screening tests.Citation11,Citation12
Sequencing of the entire genome of MAP strain K-10 in 2005 has permitted the identification of immunodominant protein antigens, which induce strong humoral or cell mediated immune responses, for subsequent inclusion in diagnostic tests or subunit based vaccines.Citation13,Citation14 Recent subunit vaccines appear to overcome many of the issues associated with whole cell strategies but nevertheless they have failed to fully protect against MAP in experimental infection models.Citation9 Huygen and Rosseels recently carried out a definitive review, entitled “Vaccination against Paratuberculosis”, which describes in detail the past and current approaches to vaccine design for paratuberculosis as well as immunodominant antigens identified prior to its publication.Citation9
Next generation animal vaccines will be complex molecular entities with multiple components tailored to generate the most potent and effective immune response. Accordingly, through comparative bioinformatic analysis of the completed MAP genome, a defined set of MAP genes encoding potentially immunodominant secreted, cytosolic and surface expressed antigens have been assembled within our laboratory. The challenge ahead is to effectively deliver these antigens in a manner that will stimulate the appropriate immune responses, a task which will require an efficient and controllable vaccine strategy. Building on the experience of previous vaccine strategies will be crucial.
Vaccine developments against other intracellular pathogens, similar to MAP, have used attenuated strains of the intracellular pathogen L. monocytogenes as effective vaccine carriers to the immune system.Citation15 These strategies are based on observations that the pathogen has both a phagosomal and cytosolic phase, facilitating the stimulation of CD4+ and CD8+ immune responses respectively.Citation15,Citation16 The infection strategy of L. monocytogenes has notable similarities to that employed by MAP but despite this, its potential as a MAP vaccine carrier has not been investigated. It is likely that this is due to concerns over the use of attenuated pathogens in vaccine development which comes with the possibility of reversion to a virulent phenotype.Citation17
Alternatively, probiotic or GRAS (generally regarded as safe) bacterial strains have also been assessed for applications in vaccine delivery strategies.Citation18,Citation19 In this field however, their potential is hampered by fragility and sensitivity toward stresses associated with formulation and environmental conditions in the gut including the presence of bile, high osmolarity, low iron or acidic environments.Citation20 The advent of patho-biotechnology, which exploits inherent mechanisms from pathogenic bacteria, has brought about novel strategies to improve the potential of these probiotic or GRAS strains for use in clinical, drug, and vaccine delivery applications.Citation17,Citation21
The main purpose of this review is to propose and evaluate the development of a novel oral subunit-vaccine against MAP and Johne disease which will utilize a patho-biotechnological approach. A Lactobacillus strain with GRAS status, harbouring an inducible expression vector encoding immunodominant MAP antigens, is to be equipped with patho-genetic elements derived from L. monocytogenes which will allow the strain to access appropriate antigen presentation pathways in order to stimulate a strong immune response. After vaccination with MAP antigens via this novel delivery platform, levels of protective efficacy will be assessed in a murine model of infection.
A successful vaccine strategy against Johne disease will require a detailed understanding of the causative agent, its mode of transmission and the associated host immune responses. The following sections will attempt to describe these aspects of MAP infection while simultaneously comparing L. monocytogenes infection, to illustrate their combined potential as part of a patho-biotechnological approach to vaccine design for Johne disease. The patho-biotechnological methodologies which have led to the present vaccine development will be discussed. The biological containment strategies to prevent the dissemination and proliferation of the vaccine strain within the environment are also outlined.
Mycobacterium avium Subspecies paratuberculosis (MAP)
MAP is shed in the feces of infected animals and can survive within the environment for up to 12 months.Citation22 Subsequently, planktonic cells may form biofilms or biofilm-like structures which could play a role in its prolonged survival, allowing the pathogen to spread horizontally to naive hosts via the fecal oral route.Citation23 Infant calves with immature immune responses are also particularly susceptible to infection via contaminated milk or colostrum on farms with a high prevalence of the disease.Citation24
Intracellular Pathogenesis
Infection with MAP takes place through the intestinal mucosa. The bacterium preferentially targets M cells present in the follicle associated epithelium (FAE) covering the continuous Peyer patches in the distal ileum of cattle.Citation25–Citation27 M cells are specialised epithelial cells which can deliver samples of foreign materials via trans-epithelial vesicular transport to intraepithelial lymphoid cells and macrophage, eliciting an immune response.Citation28 This ability to transport particles across the epithelial barrier is exploited by MAP, as well as other pathogens, to facilitate entry into the host.Citation29
The preferential targeting of M cells has been linked to the high expression of cell adhesion proteins called integrins on their luminal surface, unlike other intestinal epithelial cells.Citation29 In one study it was shown that MAP Fibronectin Attachment Protein (FAP) present on the bacterium surface forms a fibronectin bridge with these integrins on the M cells and mediates invasion of the pathogen.Citation27 However, disruption of fibronectin binding did not completely inhibit invasion of M cells indicating a fibronectin-independent mechanisms of invasion.Citation27 MAP is not restricted to this portal of entry as it has been shown to enter the small intestine of kid goats in areas without Peyer patches via enterocyte-like cells.Citation25
A MAP oxidoreductase gene (MAP 3464) was recently linked to invasion of bovine epithelial cells through the transport and folding of a MAP surface protein (MAP3985c) related to the activation of the host cell Cdc42 trigger mechanism involving cytoskeletal rearrangement and bacterial engulfment.Citation26 In this case, the MAP3464 gene product was found to be upregulated upon exposure to an MDBK epithelial cell line and was linked to invasion as a mutant MAP strain, ΔOx, was incapable of expressing the surface protein and showed a reduced invasive ability compared to the wildtype strain.Citation26
In contrast to this “trigger” mechanism used by MAP, L. monocytogenes entry to these non-phagocytic cells is mediated by a so called “zipper” mechanism characterized by intimate interaction between the pathogen and the host cell membrane leading to progressive engulfment of the bacterium.Citation30 The listerial surface protein internalin A (inlA) is involved in this attachment to, and subsequent entry of, epithelial cells which express its specific receptor, E-cadherin.Citation31 Consequently it is feasible that a GRAS (or indeed) probiotic vaccine strain equipped with this mechanism from L. monocytogenes could pass the mucosal barrier and access subepithelial immune cells of the host.
After gaining access to the subepithelial layer of the intestinal mucosa MAP is phagocytosed by subepithelial macrophages.Citation32 Once inside, the pathogen is capable of evading or misdirecting the normal killing mechanism of macrophage aimed toward clearing infection.Citation33 One study demonstrated that within bovine monocytes MAP elicited only modest production of reactive oxygen intermediates (ROI) and reactive nitrogen intermediates (RNI). Also, when compared to ingested heat-killed MAP, which displayed 94% co-localization with lysosomes, viable bacilli were able to inhibit phagolysosome fusion resulting in a reduction to 30% co-localization. The same study indicated that simultaneous intracellular multiplication and killing of MAP occurs within the phagosome.Citation34
L. monocytogenes within murine macrophage displays a similar pattern of concomitant killing and intracellular multiplication but unlike MAP is unable to prevent colocalization with lysosomes. Instead, the successful intracellular survival of L. monocytogenes is related to escape from the acidified phagosomal compartment before phagolysosome fusion has occurred.Citation35 The escape is mediated by a unique cytolysin which targets the phagosomal membrane, listeriolysin O (LLO), which is encoded by the hly gene.Citation36 LLO represents a second patho-genetic element from L. monocytogenes which could potentially be included in a recombinant MAP vaccine delivery strain.
Immune Responses to MAP and the Causation of Johne Disease
The principal goal of vaccination is to prime the immune system to facilitate a more effective and rapid immune response following infection with the pathogen, therefore understanding the interactions between the pathogen and the immune system are fundamental in the development of an effective vaccine. Paratuberculosis and Johne disease in cattle can be categorized into three distinct stages of disease or infection: (1) early infection, (2) subclinical infection and (3) clinical infection.Citation37
Herein, we briefly describe the immune responses of the host in order to explicate the pathogenesis of MAP in cattle and demonstrate the criteria necessary for effective vaccination strategies. For an in-depth analysis of the interactions between MAP and the host immune system, the reader is referred to previous reviews of particular interest.Citation37,Citation38
MAP is an intracellular pathogen and therefore the cellular immune response plays a key role in its control within the host.Citation37,Citation39 The cellular immune response is comprised of both T helper lymphocytes (CD4+) and cytolytic T lymphocytes (CTL) or killer T lymphocytes (CD8+).Citation40 T helper cells are responsible for orchestrating and directing responses, whereas CTLs are the killer cells that traffic to the site of infection and lyse infected cells.Citation40 Activated CD4+ cells can differentiate into T helper 1 (Th1) cells, which secrete proinflammatory cytokines (IFNγ and TNFα), or T helper 2 (Th2) cells, which secrete anti-inflammatory cytokines (IL-4, 5, 10 and 13).Citation40 Th1 T cells are involved in effective immune responses to intracellular pathogens by promoting microbicidal activities of macrophages. Th2 T cells promote immunoglobulin G1 (IgG1) antibody production, suppression of Th1 activity, and are likely ineffective in case of intracellular infections.Citation39
Early infection.
It is generally regarded that cell mediated immune responses dominate during early stages of the Johne' disease and are vital for control of infection.Citation41 Within the macrophage phagosome, a small proportion of MAP are lysed resulting in the presentation of antigens through the MHC class II pathway, which in turn stimulates CD4+ T cells and IFNγ productionCitation34 (see ). IFNγ is an important cytokine involved in recruitment and activation of naive macrophages, nonetheless production of IFNγ does little to cure persistently infected macrophages.Citation38 The immune system must elicit a strong CD8+ cytolytic response if it is to clear these macrophages.Citation37 Stimulation of CD8+ T cells requires antigen presentation through the MHC class I pathway which necessitates the presence of antigens in the cytosolic region, as opposed to the phagosome where MAP resides.Citation34
It has previously been demonstrated that secreted mycobacterial proteins (of up to 70 kDa in size) may freely pass into the macrophage cytoplasm. Thus MAP antigens secreted into the phagosome may escape into the cytosol and after transportation across the endoplasmic reticulum they are available for presentation via the MHC I pathway,Citation42,Citation43 (see and E). One study, in support of this, demonstrated that MAP was capable of secreting a functional low molecular weight tyrosine phosphatase into the macrophage cytoplasm which is suspected of mediating interaction with host cell substrates and interference with pathways necessary for killing the engulfed bacteria.Citation44
Maintenance of a Th1-type immune response during the early stages of infection is vital for myco-bacteriostasis.Citation37 Failure to elicit a strong enough CD8+ response to effectively clear infection results in increased tissue damage to intestinal epithelial cells (due to prolonged exposure to high levels of proinflammatory cytokines) along with an increase in bacterial loads, both of which are characteristic of subclinical infection.Citation37
Subclinical infection.
The subclinical stage of the disease can last for 2–5 years with the host presenting no clinical signs of disease, however, due to intermittent shedding in feces, MAP may be detected by fecal culture.Citation9,Citation33
Granuloma formation is an important characteristic of the subclinical phase of MAP infection and serves to sequester persistently infected macrophage to prevent pathogen spread to adjacent cells.Citation45 However, it also allows for almost unrestricted pathogen proliferation as well as providing partial protection from newly recruited CTLs. The lysis of infected macrophage by increasing levels of toxic intermediates (ROI and RNI) or CTLs causes sporadic release of MAP from granulomas and the spread of disease along the intestinal tract.Citation37
Clinical infection.
During the clinical stage of Johne disease numerous granulomas result in significant damage to the host's intestinal tract, with eventual loss of mucosal function. The animal may suffer from intermittent or persistent diarrhoea and sheds high levels of MAP into the environment. Mycobacterial-specific Th-1 immune responses are low during this stage and are replaced by non protective Th-2 responses where animals may present high antibody levels against MAP.Citation9 Animals with overt infection are usually culled from the herd immediately on detection.Citation46
Implications for an Effective MAP Vaccine
The necessity for a strong cellular Th-1 type immune response at the early stages of Johne disease, characterized by the presence of CD4+ T lymphocytes to control, and cytolytic CD8+ T lymphocytes to effectively clear the infection, has profound implications on vaccine development. A successful vaccine will require strong stimulation of both these lymphocyte subsets to induce a sufficient immunological memory which, upon challenge with MAP, can respond quickly and efficaciously.
As already mentioned L. monocytogenes displays a pattern of concomitant killing and intracellular multiplication within murine macrophage.Citation35 This facilitates the stimulation of both the CD4+ T-cell immune response (through cell destruction within the phagosome and MHC class II antigen presentation) and the CD8+ T-cell response (through multiplication within the intracytoplasmic environment and antigen presentation to the MHC class I pathway)Citation1,Citation16 (see ). Both L. monocytogenes and MAP stimulate the production of IFNγ, as well as various other proinflammatory cytokines, at an early stage of their respective infection. IFNγ is considered vital in the development of Th1-dependant acquired resistance against both of these intracellular pathogens.Citation9,Citation36
Attenuated strains of Listeria monocytogenes have previously been assessed as vaccine delivery vehicles for other intracellular pathogens, including Fransicella tularensis (the causative agent of Tularemia), and have shown promising results.Citation15 Taking this into consideration, along with the aforementioned similarities in pathogenesis, an attenuated L. monocytogenes strain would appear a potentially effective delivery vehicle in a vaccine strategy against MAP. Despite this potential, no research has been published indicating such a strategy. It is possible that this is due to concerns over the use of attenuated pathogens for vaccination, which comes with the ever present possibility of reversion to a virulent phenotype.Citation17
An intriguing alternative to using attenuated pathogens has arisen from the application of a patho-biotechnological approach to vaccine development, exploiting the potential of pathogens, but through the use of probiotic or GRAS bacterial strains.
Patho-Biotechnology
Patho-biotechnology is a term originally used by Sleator and HillCitation17 which describes the application of pathogen derived stress survival mechanisms for the design of more robust and effective probiotic cultures with improved efficacy in clinical applications, vaccine development and drug delivery platforms.Citation17,Citation20
A number of health-promoting lactic acid bacteria are currently used in clinical contexts. However, the full therapeutic potential of these probiotic bacteria is not realized as a result of their susceptibility to stresses ever present in mammalian gut such as bile, high osmolarity, low iron or acidic environments.Citation20,Citation21 Conversely, pathogenic bacteria have evolved numerous virulence mechanisms which allow them to successfully invade, persist and spread throughout a host while evading its immune responses as well as surviving for prolonged periods within the environment.Citation17 Widespread access to whole genome sequences of many pathogenic and non-pathogenic bacteria has made it possible to elucidate the genetic elements which confer these mechanisms to pathogenic strains. Subsequent application of such genetic elements to fragile probiotic strains has permitted the development of recombinant or “designer” probiotics which are sturdier and have improved tolerance to stresses.Citation20 At the forefront of patho-biotechnology, L. monocytogenes has become an important source of patho-genetic elements which are being applied to probiotic strains to improve their clinical efficacy and survival under stresses.
A Patho-Biotechnological Approach Towards Developing an Effective MAP Vaccine
Herein, we propose the development of a novel oral probiotic vaccine platform capable of expressing immunodominant MAP antigens to subepithelial macrophage thus eliciting a strong cell mediated immune against MAP using a patho-biotechnology based approach.
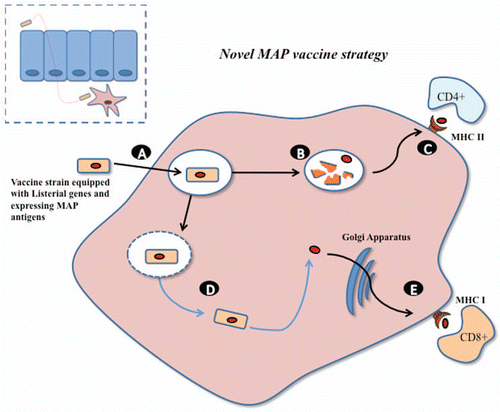
After oral ingestion the first listerial element, internalin A (inlA), will enable the Lactobacillus strain to traverse the gut wall and be engulfed by subepithelial macrophage. The second patho-genetic element, listeriolysin O (LLO), will promote escape from the phagosome and allow expression and secretion of the MAP antigens to the macrophage cytosol. While in the phagosome a certain proportion of Lactobacillus cells will be lysed releasing antigens for subsequent processing and presentation to the MHC class II pathway stimulating a strong CD4+ T-cell response. Antigens secreted within the cytosol will be processed and presented to the MHC class I pathway for stimulation of a CD8+ T-cell response. Presentation in such a manner, and the stimulation of a strong cell mediated immune response, should induce an immunological memory and provide protection against a subsequent MAP challenge in an animal model of infection (see ).
The rationale behind the application of both patho-genetic elements to the Lactobacillus delivery platform is discussed in greater detail below:
Internalin A.
As previously mentioned, internalin A (inlA) is normally used by L. monocytogenes to gain entry into mammalian intestinal epithelial cells and is critical to its pathogenicity.Citation30 In 1997, Lecuit et al. demonstrated that expression of inlA was sufficient to promote internalisation of a non invasive enterococci, as well as inert particles in the form of protein coated latex beads, into cells expressing its receptor E-cadherin (Ecad).Citation30 The interaction between InlA and E-cadherin is species specific, and although human and ruminant E-cadherin will interact with InlA, murine E-cadherin does not.Citation47 As the planned development will utilise a murine model of vaccination and experimental infection, it is our intention to use a transgenic murine line, which express humanized E-cadherin (hEcad), in the initial vaccine trials. This approach is made feasible by the fact that Lecuit et al. demonstrated that transgenic mice expressing humanized E-cadherin (hEcad) solely in enterocytes successfully interact with InlA allowing entry of L. monocytogenes to the target cells.Citation48
Another study, aimed at developing a DNA vaccine delivery strain, demonstrated that a Lactococcus lactis strain expressing inlA successfully entered intestinal cells in vivo after oral inoculation of Guinea pigs, although once internalised it was rapidly destroyed. The authors suggested that while the L. lactis inlA+ strain did not persist for long enough to induce a systemic immune response, further cloning of listeriolysin O (LLO) from L. monocytogenes would potentially increase the persistence of the recombinant strain and allow release of the L. lactis DNA content into the cytosol of host cells.Citation49
Listeriolysin O.
A 2008 study by Bahey-El-Din et al. demonstrated that a L. lactis strain expressing listeriolysin O, when delivered via intraperitoneal injection to a murine host, was capable of secreting prolonged high levels of LLO after induction and conferred protective immunity against subsequent L. monocytogenes infection.Citation50 Importantly the authors observed that an LLO-expressing L. lactis strain may have the potential to act as a platform for directing other co-expressed antigens towards the cytosolic MHC class I pathway for enhanced stimulation of the CD8+ T-cell response.
The LLO molecule itself is also a potent antigen considered to mediate a strong Th-1 type response categorized by high levels of IFNγ production.Citation36 It has been shown that LLO is capable of inhibiting Th2 immune responses by skewing differentiation of antigen specific T-cells into Th1 cells.Citation51
Stimulation of IFNγ and Th-1 type responses are widely regarded as a necessity in protection against MAP.Citation52 Therefore, the incorporation of LLO within the present vaccine will not only permit entry to the macrophage cytosol allowing presentation of antigens to CD8+ T cells, but may also act as an adjuvant to these antigens increasing the potency of the Th-1 type responses.
In contrast to the adjuvant activity of listeriolysin O (LLO) we also present an alternate strategy to be carried simultaneously, without the aid of the adjuvant, which should provide a more silent background for improved evaluation of the antigens immunostimulatory abilities.
The ruminant pathogen Listeria ivanovii secretes ivanolysin O (ILO), a cytolysin encoded by the ilo gene, which shares 80% homology in amino acid sequence to L. monocytogenes listeriolysin O (LLO).Citation53 One study, using a LLO deficient strain of L. monocytogenes expressing ILO under the control of the original hly promoter, demonstrated that ILO can functionally replace LLO in vitro for efficient phagosomal escape and intracellular multiplication. Intracellular multiplication of the ILO-expressing strain was essentially indistinguishable from that of the LLO-expressing strain.Citation53 A separate study showed that the level of IFNγ induced by a Lm ILO+ strain was significantly lower (but not null), both in vitro and in vivo, than a Lm LLO+ strain which was highly capable of inducing IFNγ. It was suggested that some structural differences between ILO and LLO possibly gave rise to the difference in level of IFNγ inducing ability.Citation36
When examined along with the current LLO+ MAP vaccine strain, a recombinant probiotic delivery platform expressing the same MAP antigens but equipped with ILO should provide valuable insights into the levels of adjuvant-like activity induced by listeriolysin (LLO) as well as giving a clearer indication of the immune responses generated to these antigens individually.
Biological Containment
The use of genetically modified organisms in veterinary medicine raises legitimate concerns about their survival and propagation in the environment and about the dissemination of antibiotic markers or other genetic modifications to other microorganisms.Citation20 Biological containment systems can be subdivided into active and passive forms. Active containment provides control through the conditional production of a compound which is toxic to the cells. Passive containment on the other hand is dependent on complementation of an auxotrophy by supplementation with either an intact gene or essential metabolite.Citation20,Citation54
We propose to address the issue of biological containment by creating conditional auxotrophic mutants of the Lactobacillus delivery strain. One of the potential targets is the thymidylate synthase (thyA) gene which is essential for growth; mutant strains only growing in the presence of added thymidine or thymine.Citation55
The choice of thyA as a target gene combines the advantages of passive and active containment systems. Thymine auxotrophy involves activation of the SOS repair system and DNA fragmentation, thereby constituting an indigenous suicide system. Thymine and thymidine growth dependence differs from most other auxotrophies in that absence of the essential component is bactericidal in the former and bacteriostatic in the latter. Thus, thyA-deficient bacteria cannot accumulate in the environment.Citation20 As previously outlined for L. lactis we propose to replace the thyA gene with a transgene (Internalin A or listeriolysin O) by double crossover using a conditionally non-replicative plasmid.Citation56
This approach addresses bio-safety concerns on a number of levels. Firstly, no resistance marker is required to guarantee stable inheritance of the transgene, thus overcoming any potential problems associated with dissemination of antibiotic resistance. Second, accumulation of the genetically modified organism in the environment is highly unlikely given that rapid death occurs upon thymidine starvation.Citation57 Finally should an intact thyA be acquired from closely related bacteria by means of homologous recombination then the transgene would be lost.
Conclusion
Examination of the complex interactions between MAP and host's immune system, the issues associated with current vaccines and the criteria generally regarded as necessary for protective immunity, has led to current proposal of a novel patho-biotechnological vaccination strategy against Johne disease. This novel vaccine consists of a Lactobacillus delivery strain with GRAS status harbouring an inducible expression vector, encoding immunodominant MAP antigens, and equipped with two patho-genetic mechanisms from the intracellular Listeria monocytogenes to enable access to subepithelial macrophages phagosomal and cytosolic compartments. Antigen presentation in this manner will stimulate both CD4+ and CD8+ T lymphocyte subsets evoking a cell mediated immune response. The protective efficacy of the selected MAP antigens as well as the effectiveness of the patho-biotechnological delivery strain will be assessed in a murine model of infection. If successful, the current vaccine development may mark a significant breakthrough in the control of Johne disease.
Abbreviations
| MAP | = | Mycobacterium avium subspecies paratuberculosis |
| GRAS | = | generally regarded as safe |
| FAE | = | follicle associated epithelium |
| FAP | = | fibronectin attachment protein |
| inlA | = | internalin A |
| ROI | = | reactive oxygen intermediates |
| RNI | = | reactive nitrogen intermediates |
| LLO | = | listeriolysin O |
| CTL | = | cytolytic T lymphocytes |
| CD4+ | = | cluster of differentiation 4 |
| CD8+ | = | cluster of differentiation 8 |
| Th1 | = | T helper 1 |
| Th2 | = | T helper 2 |
| IFNγ | = | interferon gamma |
| TNFα | = | tumour necrosis factor alpha |
| IL-4 | = | interleukin 4 |
| IgG1 | = | immunoglobulin G1 |
| MHC | = | major histocompatibility complex |
| hEcad | = | humanized epithelial cadherin |
| L. lactis | = | Lactococcus lactis |
| L. monocytogenes | = | Listeria monocytogenes |
| Lm | = | Listeria monocytogenes |
| ILO | = | ivanolysin O |
| thyA | = | thymidylate synthase A |
Figures and Tables
Figure 1 Mycobacterium avium paratuberculosis within macrophage. MAP infects by passing the mucosal barrier, preferentially via M cells, and after which it is engulfed by subepithelial macrophage (A). A number of MAP bacilli are degraded within the phagosome (B) and stimulate CD4+ immune responses via antigen presentation through the MHC class II pathway. (C) Alternatively MAP evades destruction within phagosome, through inhibition of normal killing mechanisms, and proliferates. (D) Secreted MAP proteins may pass from the phagosome to the cytosol and be subsequently available for presentation to CD8+ immune responses via the MHC class I pathway. Survival and proliferation within macrophage is a vital element in the virulence of paratuberculosis.
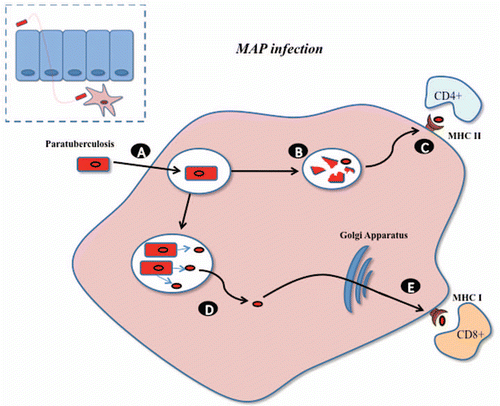
Figure 2 Infectious life cycle of L. monocytogenes (adapted from Sleator and Hill). Listeria has the ability to cross the epithelial barrier, through the expression of internalins (inlA, inlB), and after which be engulfed by sub-epithelial macrophage (A). While in the phagosome a number of cells are lysed (B) releasing antigens for subsequent presentation through the MHC class II pathway to stimulate CD4+ immune responses (C). Alternatively, Listeria escapes the phagosome using a pore forming cytolysin called listeriolysin O (D). Within the cytosol, Listerial proteins may be processed via the MHC class I pathway for stimulation of CD8+ immune responses (E). Listeria may also pass to neighboring cells through the expression of other virulence factors (F).
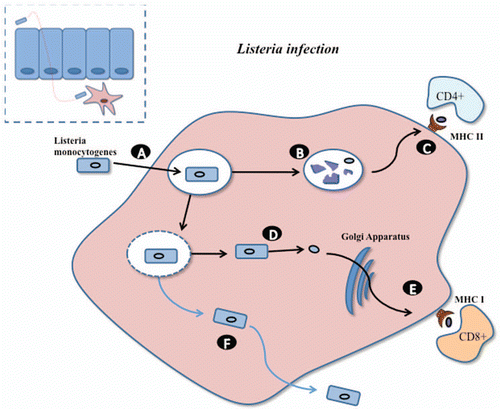
Figure 3 Novel patho-biotechnological vaccine development against Johne disease. A Lactobacillus strain, harbouring an expression vector encoding MAP antigens, will be equipped with an internalin from Listeria which will allow translocation across the epithelial barrier and subsequent phagocytosis by macrophages (A). In a similar manner to Listeria within the phagosome, a number of lactobacilli will be lysed allowing presentation of the expressed MAP antigens via the MHC class II pathway to stimulate strong CD4+ immune responses (B and C). Heterologously expressed listeriolysin O, from Listeria monocytogenes, will allow the Lactobacillus strain to escape the phagosome. (D) Expression of MAP antigens within the macrophage cytosol will stimulate strong CD8+ immune responses through the MHC class I pathway (E).
Acknowledgements
The author's acknowledge the financial assistance of the Irish Government through funding by a Food Institutional Research Measure (FIRM) grant.
References
- Chacon O, Bermudez LE, Barletta RG. Johne's disease, inflammatory bowel disease, and Mycobacterium paratuberculosis. Annu Rev Microbiol 2004; 58:329 - 363
- Sohal JS, Singh SV, Singh PK, Singh AV. On the evolution of ‘Indian Bison type’ strains of Mycobacterium avium subspecies paratuberculosis. Microbiol Res 2010; 65:163 - 171
- Sleeman JM, Manning EJ, Rohm JH, Sims JP, Sanchez S, Gerhold RW, et al. Johne's disease in a free-ranging white-tailed deer from Virginia and subsequent surveillance for Mycobacterium avium subspecies paratuberculosis. J Wildl Dis 2009; 45:201 - 206
- Harris NB, Barletta RG. Mycobacterium avium subsp. paratuberculosis in Veterinary Medicine. Clin Microbiol Rev 2001; 14:489 - 512
- Ott SL, Wells SJ, Wagner BA. Herd-level economic losses associated with Johne's disease on US dairy operations. Prev Vet Med 1999; 40:179 - 192
- Barrett D, Good M, Hayes M, More SJ. The economic impact of Johne's disease in an Irish dairy herd: A case study. Irish Vet J 2006; 59:282 - 288
- Hermon-Taylor J. Mycobacterium avium subspecies paratuberculosis, Crohn's disease and the Doomsday scenario. Gut Pathog 2009; 1:15
- Scanu AM, Bull TJ, Cannas S, Sanderson JD, Sechi LA, Dettori G, et al. Mycobacterium avium subspecies paratuberculosis infection in cases of irritable bowel syndrome and comparison with Crohn's disease and Johne's disease: common neural and immune pathogenicities. J Clin Microbiol 2007; 45:3883 - 3890
- Rosseels V, Huygen K. Vaccination against paratuberculosis. Expert Rev Vaccines 2008; 7:817 - 832
- Collins MT. Clinical approach to control of bovine paratuberculosis. J Am Vet Med Assoc 1994; 204:208 - 210
- Nedrow AJ, Gavalchin J, Smith MC, Stehman SM, Maul JK, McDonough SP, et al. Antibody and skin-test responses of sheep vaccinated against Johne's Disease. Vet Immunol Immunopathol 2007; 116:109 - 112
- Mackintosh CG, Labes RE, Thompson BR, Clark RG, de Lisle GW, Johnstone PD, et al. Efficacy, immune responses and side-effects of vaccines against Johne's disease in young red deer (Cervus elaphus) experimentally challenged with Mycobacterium avium subsp paratuberculosis. N Z Vet J 2008; 56:1 - 9
- Li L, Bannantine JP, Zhang Q, Amonsin A, May BJ, Alt D, et al. The complete genome sequence of Mycobacterium avium subspecies paratuberculosis. Proc Natl Acad Sci USA 2005; 102:12344 - 12349
- Santema W, Overdijk M, Barends J, Krijgsveld J, Rutten V, Koets A. Searching for proteins of Mycobacterium avium subspecies paratuberculosis with diagnostic potential by comparative qualitative proteomic analysis of mycobacterial tuberculins. Vet Microbiol 2009; 138:191 - 196
- Jia Q, Lee BY, Clemens DL, Bowen RA, Horwitz MA. Recombinant attenuated Listeria monocytogenes vaccine expressing Francisella tularensis IglC induces protection in mice against aerosolized Type A F. tularensis. Vaccine 2009; 27:1216 - 1229
- Starks H, Bruhn KW, Shen H, Barry RA, Dubensky TW, Brockstedt D, et al. Listeria monocytogenes as a vaccine vector: virulence attenuation or existing antivector immunity does not diminish therapeutic efficacy. J Immunol 2004; 173:420 - 427
- Sleator RD, Hill C. ‘Bioengineered Bugs’—a patho-biotechnology approach to probiotic research and applications. Med Hypotheses 2008; 70:167 - 169
- Pouwels PH, Leer RJ, Shaw M, Heijne den Bak-Glashouwer MJ, Tielen FD, Smit E, et al. Lactic acid bacteria as antigen delivery vehicles for oral immunization purposes. Int J Food Microbiol 1998; 41:155 - 167
- Shi D, Song Y, Li YJ. Progress on Lactococcus lactis expressing heterologous antigens as live mucosal vaccines. Wei Sheng Wu Xue Bao 2006; 46:680 - 683
- Sleator RD, Hill C. Improving probiotic function using a pathobiotechnology approach. Gene Ther Mol Biol 2007; 11:269 - 274
- Sleator RD, Hill C. New frontiers in probiotic research. Lett Appl Microbiol 2008; 46:143 - 147
- Whittington RJ, Marshall DJ, Nicholls PJ, Marsh IB, Reddacliff LA. Survival and dormancy of Mycobacterium avium subsp. paratuberculosis in the environment. Appl Environ Microbiol 2004; 70:2989 - 3004
- Wu CW, Schmoller SK, Bannantine JP, Eckstein TM, Inamine JM, Livesey M, et al. A novel cell wall lipopeptide is important for biofilm formation and pathogenicity of Mycobacterium avium subspecies paratuberculosis. Microb Pathog 2009; 46:222 - 230
- Baptista FM, Nielsen SS, Toft N. Association between the presence of antibodies to Mycobacterium avium subspecies paratuberculosis and somatic cell count. J Dairy Sci 2008; 91:109 - 118
- Sigurdardóttir OG, Bakke-McKellep AM, Djønne B, Evensen O. Mycobacterium avium subsp. paratuberculosis enters the small intestinal mucosa of goat kids in areas with and without Peyer's patches as demonstrated with the everted sleeve method. Comp Immunol Microbiol Infect Dis 2005; 28:223 - 230
- Alonso-Hearn M, Patel D, Danelishvili L, Meunier-Goddik L, Bermudez LE. The Mycobacterium avium subsp. paratuberculosis MAP3464 gene encodes an oxidoreductase involved in invasion of bovine epithelial cells through the activation of host cell Cdc42. Infect Immun 2008; 76:170 - 178
- Secott TE, Lin TL, Wu CC. Mycobacterium avium subsp. paratuberculosis fibronectin attachment protein facilitates M-cell targeting and invasion through a fibronectin bridge with host integrins. Infect Immun 2004; 72:3724 - 3732
- Neutra MR. Current concepts in mucosal immunity. V Role of M cells in transepithelial transport of antigens and pathogens to the mucosal immune system. Am J Physiol 1998; 274:785 - 791
- Sansonetti PJ, Phalipon A. M cells as ports of entry for enteroinvasive pathogens: mechanisms of interaction, consequences for the disease process. Semin Immunol 1999; 11:193 - 203
- Lecuit M, Ohayon H, Braun L, Mengaud J, Cossart P. Internalin of Listeria monocytogenes with an intact leucine-rich repeat region is sufficient to promote internalization. Infect Immun 1997; 65:5309 - 5319
- Mengaud J, Ohayon H, Gounon P, Mege R-M, Cossart P. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell 1996; 84:923 - 932
- Momotani E, Whipple DL, Thiermann AB, Cheville NF. Role of M cells and macrophages in the entrance of Mycobacterium paratuberculosis into domes of ileal Peyer's patches in calves. Vet Pathol 1988; 25:131 - 137
- Basler T, Geffers R, Weiss S, Valentin-Weigand P, Goethe R. Mycobacterium avium subspecies induce differential expression of pro-inflammatory mediators in a murine macrophage model: evidence for enhanced pathogenicity of Mycobacterium avium subspecies paratuberculosis. Immunobiology 2008; 213:879 - 888
- Woo SR, Heintz JA, Albrecht R, Barletta RG, Czuprynski CJ. Life and death in bovine monocytes: the fate of Mycobacterium avium subsp. paratuberculosis. Microb Pathog 2007; 43:106 - 113
- de Chastellier C, Berche P. Fate of Listeria monocytogenes in murine macrophages: evidence for simultaneous killing and survival of intracellular bacteria. Infect Immun 1994; 62:543 - 553
- Hara H, Kawamura I, Nomura T, Tominaga T, Tsuchiya K, Mitsuyama M. Cytolysin-dependent escape of the bacterium from the phagosome is required but not sufficient for induction of the Th1 immune response against Listeria monocytogenes infection: distinct role of Listeriolysin O determined by cytolysin gene replacement. Infect Immun 2007; 75:3791 - 3801
- Coussens PM. Mycobacterium paratuberculosis and the bovine immune system. Anim Health Res Rev 2001; 2:141 - 161
- Sohal JS, Singh SV, Tyagi P, Subhodh S, Singh PK, Singh AV, et al. Immunology of mycobacterial infections: with special reference to Mycobacterium avium subspecies paratuberculosis. Immunobiology 2008; 213:585 - 598
- Koets A, Rutten V, Hoek A, van Mil F, Müller K, Bakker D, et al. Progressive bovine paratuberculosis is associated with local loss of CD4(+) T cells, increased frequency of gamma delta T cells, and related changes in T-cell function. Infect Immun 2002; 70:3856 - 3864
- Esser MT, Marchese RD, Kierstead LS, Tussey LG, Wang F, Chirmule N, et al. Memory T cells and vaccines. Vaccine 2003; 21:419 - 430
- Bannantine JP, Bayles DO, Waters WR, Palmer MV, Stabel JR, Paustian ML. Early antibody response against Mycobacterium avium subspecies paratuberculosis antigens in subclinical cattle. Proteome Sci 2008; 28:5 - 6
- Teitelbaum R, Cammer M, Maitland ML, Freitag NE, Condeelis J, Bloom BR. Mycobacterial infection of macrophages results in membrane-permeable phagosomes. Proc Natl Acad Sci USA 1999; 96:15190 - 15195
- Lilenbaum W, Marassi CD, Oelemann WMR. Paratuberculosis: an update. Braz J Microbiol 2007; 38:580 - 590
- Bach H, Sun J, Hmama Z, Av-Gay Y. Mycobacterium avium subsp. paratuberculosis PtpA is an endogenous tyrosine phosphatase secreted during infection. Infect Immun 2006; 74:6540 - 6546
- Lei L, Plattner BL, Hostetter JM. Live Mycobacterium avium subsp. paratuberculosis and a killed-bacterium vaccine induce distinct subcutaneous granulomas, with unique cellular and cytokine profiles. Clin Vaccine Immunol 2008; 15:783 - 793
- Whitlock RH, Buergelt C. Preclinical and clinical manifestations of paratuberculosis (including pathology). Vet Clin North Am Food Anim Pract 1996; 12:345 - 356
- Lecuit M, Dramsi S, Gottardi C, Fedor-Chaiken M, Gumbiner B, Cossart P. A single amino acid in E-cadherin responsible for host specificity towards the human pathogen Listeria monocytogenes. EMBO J 1999; 18:3956 - 3963
- Lecuit M, Vandormael-Pournin S, Lefort J, Huerre M, Gounon P, Dupuy C, et al. A transgenic model for listeriosis: role of internalin in crossing the intestinal barrier. Science 2001; 292:1722 - 1725
- Guimarães VD, Gabriel JE, Lefívre F, Cabanes D, Gruss A, Cossart P, et al. Internalin-expressing Lactococcus lactis is able to invade small intestine of guinea pigs and deliver DNA into mammalian epithelial cells. Microbes Infect 2005; 7:836 - 844
- Bahey-El-Din M, Casey PG, Griffin BT, Gahan CG. Lactococcus lactis-expressing listeriolysin O (LLO) provides protection and specific CD8(+) T cells against Listeria monocytogenes in the murine infection model. Vaccine 2008; 26:5304 - 5314
- Yamamoto K, Kawamura I, Tominaga T, Nomura T, Kohda C, Ito J, et al. Listeriolysin O, a cytolysin derived from Listeria monocytogenes, inhibits generation of ovalbumin-specific Th2 immune response by skewing maturation of antigen-specific T cells into Th1 cells. Clin Exp Immunol 2005; 142:268 - 274
- Stabel JR. Transitions in immune responses to Mycobacterium paratuberculosis. Vet Microbiol 2000; 77:465 - 473
- Frehel C, Lety MA, Autret N, Beretti JL, Berche P, Charbit A. Capacity of ivanolysin O to replace listeriolysin O in phagosomal escape and in vivo survival of Listeria monocytogenes. Microbiology 2003; 149:611 - 620
- Steidler L, Rottiers P. Therapeutic drug delivery by genetically modified Lactococcus lactis. Ann N Y Acad Sci 2006; 1072:176 - 186
- Steidler L. Genetically engineered probiotics. Best Prac Res Clinical Ga 2003; 17:861 - 876
- Steidler L, Neirynck S, Huyghebaert N, Snoeck V, Vermeire A, Goddeeris B, et al. Biological containment of genetically modified Lactococcus lactis for intestinal delivery of human interleukin 10. Nat Biotechnol 2003; 21:785 - 789
- Ahmad SI, Kirk SH, Eisenstark A. Thymine metabolism and thymineless death in prokaryotes and eukaryotes. Annu Rev Microbiol 1998; 52:591 - 625