Abstract
Volatile fuel costs, need to reduce greenhouse gas emissions, and fuel security concerns are driving efforts to produce sustainable renewable fuels and chemicals. Petroleum comes from sunlight, CO2, and water converted via a biological intermediate into fuel over a several million year timescale. It stands to reason that using biology to short-circuit this time cycle offers an attractive alternative-but only with relevant products at or below market prices. The state of the art of biological engineering over the past five years has progressed to allow for market needs to drive innovation rather than trying to adapt existing approaches to the market. This report describes two innovations using synthetic biology to dis-intermediate fuel production. LS9 is developing a means to convert biological intermediates such as cellulosic hydrolysates into drop-in hydrocarbon product replacements such as diesel. Joule Unlimited is pioneering approaches eliminate feedstock dependency by efficiently capturing sunlight, CO2, and water to produce fuels and chemicals. The innovations behind these companies are built with the market in mind, focused on low cost biosynthesis of existing products of the petroleum industry. Through successful deployment of technologies such as those behind LS9 and Joule, alternative sources of petroleum products will mitigate the many of the issues faced with our petroleum-based economy.
Introduction
The engineering of microorganisms for fuel production is experiencing a renaissance given the abundant need to develop alternative sources of fuel. Recent innovations, such as those pioneered in the development of LS9 and Joule Unlimited, are helping to reshape the thinking about fuel production from recombinant organisms. I founded both of these companies through Flagship VentureLabs to solve fundamental bottlenecks in fuel production, thereby increasing efficiencies and reducing costs.
Flagship VentureLabs was founded to streamline the process by which new innovation could be conceived, funded and brought to market outside of typical constraints. Here, breakthrough technologies are developed to match large unmet needs in healthcare and sustainability markets and transformed into proprietary start-up companies. To date, over 20 start-up companies have been formed within the Labs, including LS9 and Joule Unlimited. These first-in-class companies, conceptualized and founded by members of the Flagship VentureLabs team, are started within its Cambridge offices. Fundamentally, we remove the constraints from the typical elements of traditional ecosystems and harness them all under one roof, with the common goal of the betterment of humankind through innovation and entrepreneurship. This results in conceiving of new ideas and forming companies several years before more mainstream investors realize an emerging market opportunity is likely to be compelling.
A principal focus of mine at Flagship VentureLabs has been using techniques of biological engineering to dis-intermediate processes to maximize efficiency. Fuel production has been a key focus for this approach. Fuel is effectively the energy of the sun captured in chemical form. Traditional petrol fuels are fundamentally solar radiation and CO2 converted into hydrocarbons through photosynthesis millions of years ago, with the biological products modified to their state in natural deposits through a complex process involving high temperature and pressure over time. Current approaches to supplant petroleum effectively attempt to shortcut this process to make fuels over shorter periods of time.
The idea to leverage biology to enhance fuel production is not new. Efforts to produce cellulosic ethanol have been afoot for more than 30 years. In fact, several of the first biotechnology companies, such as Amgen, Genentech and Biogen, contemplated using recombinant techniques to produce fuels. Over the past 30+ years, breakthroughs in genomic research, understanding the circuit-like connectivity of intracellular systems, and genetic engineering capabilities have provided new capabilities in the targeted manipulation of cells to achieve specific functionsCitation1 such that now, the same fundamental questions can be asked with a new lens as to what is achievable. Furthermore, the progressive advancements over the last 30+ years are enabling faster translation from conception to commercial productivities—akin to Moore's law for transistors—with times decreasing three-fold every ten years.
The predominant approach today for renewable fuel synthesis is the production of biofuels—or biomass-derived fuels. In general, sunlight and CO2 are captured by plants. These plants, grown over one-to-ten years, are harvested to yield sugars of various forms. Subsequently, fermentative or non-fermentative approaches are used to convert this sugar into something that either is or can be processed into a fuel. Traditional approaches have focused on ethanol approaches. Recently, however, LS9 has pioneered the efficient bioconversion of sugar into hydrocarbons, including diesel. Based on cutting edge biological engineering approaches, LS9 was built around leveraging maximally efficient biological pathways focused not only on maximized production efficiency but also on minimized need for extremely expensive post-processing steps, which together, enable the lowest cost production of drop-in fuels as well as of low cost chemicals.
Algal biofuels are an effort to further streamline fuel production. Here, sunlight and CO2 are captured by a photosynthetic organism typically selected for rapid growth and membrane accumulation of lipids. After a period of growth, the organisms are harvested and dehydrated, the membrane separated and the molecules converted into biodiesel—an alkyl ester, chemically distinct from mass market fuels—through one of several processes. This approach, however, fundamentally suffers from thermodynamic limitations that make economic viability difficult, if not impossible, to achieve. An emerging approach to truly streamline renewable fuel synthesis is that of Solar Fuels, pioneered by Joule Unlimited. In this process, photosynthetic microorganisms are engineered to capture sunlight and CO2 and directly synthesize and secrete programmed end-products, thus simplifying the petroleum process for any given fuel or chemical to a single step.
I will discuss the fundamental innovations behind these two companies as well as their transformative potential.
LS9: Ultra Clean Renewable Diesel
Heterotrophic organisms have been used for millennia to produce products of interest, starting with various fermented beverages. In some of these oldest processes, Saccharomyces cervisiae was used as the organism of choice primarily because of its innate capabilities to produce a desired end-product: ethanol. Even in recent years, S. cervisiae has remained the organism of choice for the production of corn ethanol. Recent advancements, however, have expanded the repertoire of organisms, as well as the products they can make such as Escherichia coli engineered to produce ethanolCitation2 and E. coli engineered to produce plastics.Citation3 The incorporation of different host organisms as well as designer pathways, whether for augmenting feedstock breadth or for preferred output chemicals, has been enabled by continued innovations in recombinant techniques over the last 30+ years.
Within the past decade, however metabolic engineering and synthetic biology capabilities have been extended exponentially now to allow one to ask open-ended questions based on what the ultimate goal is. LS9 was founded based on the question of “how can we produce a scalable, low cost, infrastructure compatible biofuel?”
Simply put, the ideal fuel to be produced from biology would be diesel given its high energy density and its use throughout the world as a primary transportation fuel. This biologically produced diesel needs to be produced in the most economical manner to be competitive in a low cost commodity market. As a result, a means to bioconvert sugar into diesel needed to be created, incorporating not just the biological pathway, but also the process. In order to achieve this, four key requirements were set: the ability to be feedstock agnostic (i.e., use any form of sugar), the most efficient biological pathway, a means for low cost separation and a product that did not require additional processing. In the initial phases of LS9, a process to define a means to achieve all of these goals simultaneously was undertaken. The solution that emerged was the use of a well understood organism for engineering, E. coli, to serve as a host for sugar metabolism genes, key pathway operon and secretion machinery in order to efficiently produce of end-products not requiring additional processing prior to industrial use.
Fats are the preferred energy storage compound in many organisms. Not surprisingly, the pathway to produce them—fatty acid biosynthesis—is over 90% energy efficient. It stands to reason, therefore, that this pathway could produce energy storage molecules for low cost fuel, with appropriate modifications to make fungible products. After designing several pathways that could yield diesel or a precursor, in silico analysis was performed, confirming the predicted higher efficiency of this pathway relative to others.
As an example of another pathway evaluated, the isoprenoid biosynthesis pathway can also yield diesel precursors but is fatally flawed. Two isoprenoid pathways have been described to produce a fuel precursor, the mevalonate and deoxyxylulose pathways,Citation4 both of which have energy efficiencies of only 60–70%. This is not surprising because this pathway is used in nature to make vitamins, co-factors and other molecules focused on specific enzymatic or related events rather than energy storage. The products of these two pathways, isopentenyl pyrophosphate and dimethylallyl pyrophosphate respectively, are then linked head-to-tail to form monoterpene, sesquiterpene, farnesylphosphate or geranylgeranyl pyrophosphate. The direct pathway products as well as farnesylphosphate and geranylgeranyl pyrophosphate then require additional processing themselves to produce fuel precursors,Citation5 which then require chemical modifications to yield a fuel. This pathway has been the subject of much exploration to produce biofuels.Citation6–Citation8 The combination of markedly reduced biosynthesis efficiency and added cost associated with needed additional processing pose significant challenges to this approach.
The high efficiency associated with fatty acid biosynthesis makes it a preferred means to produce diesel. In this pathway, fatty acids are activated with coenzyme A or acyl carrier protein (ACP), producing fatty acyl-CoA or fatty acyl-ACP,Citation9 which serve as the starting molecules for fuel synthesis. Enzymatic pathways for diesel, as well as for a series of other fuels and chemicals, can be added from this point in biosynthesis. Beyond diesel (alkane), these can include fatty acid methyl esters (other fuels and chemicals) also known as biodiesel, olefins, fatty alcohols and many others. Each of these products can be synthesized directly in the cell using fatty acid biosynthesis coupled with specific engineering of pathways to make the desired compound.
An efficient cellular chassis is essential to minimize cost of products. This chassis must be optimized to maximize flux of carbon from input sugar though the fatty acid biosynthesis pathway and to enable secretion. The flux is optimized by directing carbon from sugar, which gets taken into central metabolism, to the fatty acid biosynthesis pathway. For example, eliminating γ-oxidation through fadD and fadE knockouts blocks the first two steps of the β-oxidation pathway, yielding a three-to-fourfold increase in end-product.Citation10 Secretion serves two purposes. First, the accumulation of acyl-ACP inhibits fatty acid biosynthesis. Expression of a leaderless version of TesA hydrolyzes the acyl-ACPs, reduces inhibition of fatty acid biosynthesis and enables secretion of fatty acids (in the absence of a product-specific pathway).Citation10–Citation12 The selectivity of this version of TesA has a preference for C14 fatty acids, though can produce a range across the commercially relevant spectrum.Citation10 Second, secretion offers a fundamental process advantage: continuous operations. With a continuous process, centrifugation is sufficient to achieve product separation, as opposed to much more complex and costly methods associated with batch processes for similar types of products, such as those that have been used for algal oil isolation. Enabling secretion therefore simultaneously replaces the batch processing of biofuels with a continuous one and supplants expensive separation techniques such as distillation with a low cost approach.
Through gene discovery, pathway engineering and metabolic modeling, LS9 has achieved first-in-class capabilities to produce a litany of fuels and chemicals, all of which are secreted from the cell in final form, using the common chassis. Alkanes are a preferred compound as an alternative diesel source. Adding one such pathway to the engineered chassis allows for bio-based diesel production. Other compounds are produced by similarly understanding the biochemical pathway for synthesis and adding the appropriate genes to the chassis. Two biological mechanisms have been proposed: decarbonylation of a fatty aldehyde intermediate yielding an odd-numbered carbon chain (which are similar to those found in nature) or the direct reduction of fatty alcohols.Citation13,Citation14 Biodiesel production, as described in Steen et al.Citation10 can be achieved using a specific acyltransferase in conjunction with an ethanol production pathway. The acyltransferase allows for the production of fatty acids, and the ethanol pathway (pdc and adhB) provides for an endogenous source of the alcohol necessary for condensation into the alkyl ester. Olefins can be synthesized through recently discovered genes that can allow for the head-to-head condensation of two fatty acids.Citation15 Finally, fatty alcohols can be produced using eukaryotic fatty acyl- CoA reductases.Citation16,Citation17 Of particular note, each of these pathways leads directly to a commercially relevant end-product. No additional processing is necessary for any of the products. This feature is unique to the fatty acid biosynthesis pathway, as the hydrocarbon base reflects the chemical nature of the fuels and chemicals to be produced.
Just as the chassis can be engineered for a specific product, so too can it be for sugar uptake. While simple sugars such as glucose from sugar cane can be used readily with the LS9 chassis, land-use pressures, cost-considerations and import tariffs put pressures to expand the base of inputs. Current approaches use exogenous enzymes to liberate sugar cellulosic biomass. Engineering genes with a similar functionality into the chassis can enable consolidated bioprocessing with endogenous production of glycosyl hydrolases, reducing process costs.Citation18 Expression of hemicellulases—a xylanase (Xsa) from Bacteroides ovatus and an endoxylanase catalytic domain (Xyn10B) from Clostridium stercorarium—hydrolyzes hellicellulose to xylose, which can then be taken up and incorporated into central metabolism in E. coli.Citation10,Citation19,Citation20
The combination of the various metabolic engineering capabilities produces a designer biocatalyst that is feedstock agnostic, exhibits highly efficient conversion, uses a one-step process and yields products with drop-in compatibility. This amalgamation of key features also produces a diesel, for example, with minimal sulfur, significant life-cycle reductions in CO2 emissions, minimal benzene, while retaining all of the functional features of petroleum-derived diesel including cetane rating, oxidative stability and cloud point of diesel. Furthermore, the costs of production are competitive without subsidy. By combining all of these factors, LS9's biosynthetic pathway represents a powerful means to produce commercially relevant products at commercially relevant costs.
Joule Unlimited: Renewable Solar Fuels
The dependency of output cost on feed-stock prices is an intrinsic challenge faced by all heterotrophic organisms used in the production of commodities. Efforts to minimize this dependency have focused on direct uptake of inputs by an organism that can be used to make an intermediate or end-product. As carbon is the central currency of biomaterial production, organisms that can take up and use some form of carbon are desirable. Photosynthesis is nature's solution to assimilating carbon, doing so in the form of CO2, using solar energy to achieve the process.
Algal biofuels have attempted to use CO2 as a carbon source industrially, but face a series of intrinsic limitations. Significant effort and funding was put into exploring algal biofuels between 1976 and 1996 under the Department of Energy's Aquatic Species program.Citation21 Ultimately, the study concluded that photosynthesis could support viable fuel processes, but needed key innovations to achieve viability. Fundamental issues included dependency on batch processing, low efficiency maximums associated with membrane-accumulating products and high costs of separations. These limitations have been validated by both theoretical and empirical studies.Citation22,Citation23 Open algal ponds are further challenged by an inability to use genetically modified organisms, as well as by invading species.Citation21,Citation24
What had fundamentally not been addressed by algae was how to shortcut the entirety of petroleum synthesis into a single step biological process. This specifically involves an organism that can use photosynthesis and produce an end-product that is directly secreted. Joule Unlimited was founded to specifically solve this challenge using an engineering based methodology in order to design sophisticated biological capabilities using multi-scale system analysis. While scientifically interesting in its own right, single-step fuel or chemical synthesis is viable only when practical issues for deployment are taken into account. As such, Joule Unlimited also sought to develop its direct-to-end product process to specifically not deplete agriculture land, not have a requirement for fresh water, produce fungible products with full infrastructure compatibility, and have a cost that would, without subsidy, beat fossil fuel equivalents.
The fundamental biology of photosynthesis must be leveraged and optimized to create an industrial process. The maximum activity of photosynthetic efficiency is governed by broad environmental conditions as well as providing for a means in which photosynthetic activity can be utilized in a productive manner. Light energy, which provides the driving force for photosynthesis, scales two-dimensionally, with a given number of photons striking an area per unit time. Ground level insolation rates have been determined by the National Renewable Energy Laboratory including not only total intensity, but specifically including photosynthetically active radiation (approximately 39% of photonic energy striking the ground).Citation25,Citation26 Oxygenic photosynthesis uses light energy through the Z-scheme where water is oxidized, NADP+ is reduced to NADPH and ADP is generated. An 8 photon-cycle of the Z-scheme, which generates 2 NADPH and 3 ATP, is required to fix one CO2.Citation27 The nature of this biology points to a series of elements needed to create an industrially viable process: optimized light exposure, tailored CO2: light input ratios and biological system design to efficiently use the products of photosynthesis.
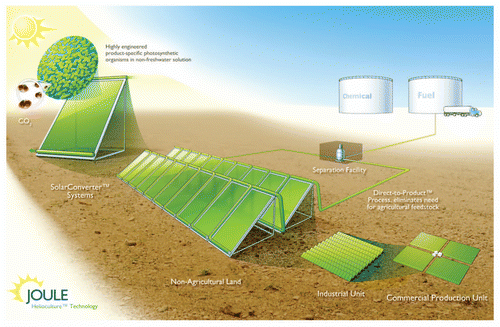
Nature has designed an effective system to capture CO2. The net efficiency of the photosynthetic machinery, as well as that of the downstream biological processes is dependent on the system in which the reaction is occurring. For efficient areal capture, a reactor design that optimizes culture density, gas-liquid mass transfer, temperature and light exposure is required. Additionally, a biological system designed to minimizes losses associated with CO2 reduction, mitochondrial respiration, photorespiration, cellular maintenance, process cycle time and non-product synthesis is necessary. These two components must be co-developed to ensure that the variables are co-optimized. A schematic of the Joule Unlimited approach is provided in . Joule has developed a reactor, the Solar Converter, which is designed to allow for key biologies to occur while harnessing the modular scalability seen with solar panels. The reactor is designed to use CO2 to drive mixing, facilitating both gas-liquid mass transfer and idealized light exposure time cycles. The reactor is made of very low cost materials with proven outdoor life-spans with a means for passive thermal management. The modular design is central to reducing the risk associated with scaling up an otherwise complex process. Once a small number of reactors is used to validate operations, larger numbers, which by definition use an identical process, will be known to work prior to deployment. Larger numbers of future ones will be known to work in a similar manner. This substantially de-risks deployment and allows for flexible plant sizing.
System based genome engineering is used in order to design a biological system capable of leveraging the Solar Converter. As a starting point, a cell with the right features—viability in the appropriate temperature range, growth in non-fresh water, absence of biological properties that would prevent genetic engineerability, sufficiently fast doubling time, membranes that could allow for secretion—had to be selected. This entailed a blind screen of several hundred organisms from commercial, public, and proprietary sources, which produced only a handful of qualified potential host organisms. Unfortunately, however, traditional genetic engineering techniques had not been well established in these key organisms. Therefore, the tools to accomplish large-scale genome engineering needed to be developed. Using synthetic biology capabilities, Joule Unlimited first recapitulated the industrialization and technique development over 50 years in E. coli in just two years. The cell most amenable to technology development was selected. This provided a fundamental tool box essential to design an optimized photosynthetic production host.
Using these techniques the favored host organism was built to serve as a chassis by engineering a series of biological and process functionalities in order to maximize efficiency. First, metabolism was diverted to product synthesis. Photosynthetic organisms naturally use sunlight, CO2 and water, for growth and maintenance, with O2 as a byproduct. Through a systematic re-organization and re-regulation of central metabolism, assimilated carbon can be driven to end-products through key selected off-take points. This allows for up to 95% of photosynthetic activity to be driven towards product synthesis with only 5% towards growth and maintenance. Second, cells were engineered with the ability to produce key products. The chassis is developed such that product specific operons, which must be tailored to the host organism, can be dropped in to allow for synthesis. All products selected are fungible end-products used industrially today. The products fall into two broad categories: volatile and organic. Volatile products, such as ethanol, are passively released from the cells. Aqueous products, such as diesel, require the engineering of a transporter for secretion. Product selection is of intriguing importance in photosynthetic organisms in that different products have distinct photon requirements for CO2 fixation as a function of the biosynthetic pathway's needs for ATP and/or NADPH. Understanding these requirements is essential to maximizing yield. At low ratios, reducing power (with product-to-product variability) is limiting, imposing a limitation on the yield potential. Higher ratios require the development of a shunt for the product of excess (ATP or NADPH) in order to have the potential for 100% theoretical yield. These engineered capabilities alone eliminate the dependency on batch process, increase biological efficiencies and yield non-membrane-accumulating products, overcoming several of the traditional limitations of photosynthetic organisms.
Incorporating the biology with the Solar Converter allows for the construction of a fully integrated system. Cells are run under a semi-continuous process such that only 5% of time is dedicated to culture growth and 95% to production. The maximum culture density is selected such that, given the depth of the reactor, mixing occurs readily, dark volume is minimized, but light is exhausted, thus optimizing photon utilization. Product separations are built into the process as well such that secreted end-products are continuously removed from the system. Volatile products can be collected from the vapor phase in a simplified distillation-like process, while aqueous products can be captured by centrifugation of media.
Effectively, this approach creates a photosynthetic system where carbon flux is maximally diverted to a synthetic pathway that produces and secretes drop-in end-products under conditions of limited organism growth. Genome engineering is used, therefore, to create a system wherein previously unengineerable autotrophs function like industrialized heterotrophic organisms whose phases of growth and production are uncoupled to maximize productivity without dependency on sugar. The impact of these changes are clear: rather than a theoretical maximum productivity for algal biofuels of 2,000 gallons per acre per year, the combination of optimized heterotroph-like biology with a tailored reactor system leads to unprecedented areal productivities of 25,000 gallons ethanol/acre/year or 15,000 gallons diesel/acre/year.
Through a series of steps, Joule Unlimited has therefore created a chassis easily adapted to a variety of outputs that maximizes solar energy capture, CO2 fixation and organism productivity. The resultant productivities are sufficiently high that the system can incur the capital cost associated with the reactors, and still be competitive with crude oil prices of less than $30/barrel. Furthermore, because CO2 is a key direct input, fuels made by Joule reduce life cycle greenhouse gas emissions by up to 90%.
Conclusions
Breakthrough innovations are necessary to escape our dependency on fossil fuels and their associated problems. Biological engineering has come of age to allow for cell systems to be designed for a purpose. Full realization of the power of cell engineering depends on identifying the key market needs and demands, as well as design a biological suitable approach to meet them.
The fundamental approach LS9 and Joule Unlimited have taken is to use techniques of biological engineering to dis-intermediate the process from which sunlight and CO2 are converted into petrochemical products. LS9 has taken key products used in the petroleum industry, such as diesel and back-engineered means to produce it from near-term-available biological intermediates, such as sugar. Joule Unlimited has similarly back-engineered methods to eliminate feedstock volatility by using sunlight and CO2 as inputs to generate the currency of central metabolism. These two approaches together represent paths to disintermediate petroleum production.
Outside of periods of government intervention, fuel markets are driven by price and purity, supply and demand. Markets thus require products where a specific use exists and are available at reasonable cost. Fundamentally, any alternative source of fuel with aspirations for significant adoption must therefore produce products meeting industry specifications at competitive costs. Competitiveness must not depend on time-limited subsidies or the unclear potential for carbon offsets, and must be validated without requiring enormous amounts of capital along the way. These market requirements pose the need for additional considerations in designing a biological process: continuous processes are essential, costly separations and post-processing steps must be eliminated and efficient scalability must be a design constraint. Only by designing biologically engineered systems with the market needs in mind—finding the intersection between market pull and the potential for technology solution—can meaningful bio-based alternatives to fossil fuels become a reality.
Figures and Tables
Figure 1 Schematic of Joule Unlimited Helioculture system. Proprietary organisms are engineered to convert sunlight, CO2 and non-fresh water to industrially relevant end-products. The engineered organisms are housed within a Solar Converter, which represents a single rector unit. Operational modules are constructed by interconnecting several Solar Converters. This allows for reaction condition control as well as continuous separations. Figure from www.jouleunlimited.com/why-solar-fuel/how-it-works
Acknowledgements
I would like to thank the many scientists, engineers and advisors who have worked on making the innovations behind LS9 and Joule Unlimited a reality.
References
- Khalil AS, Collins JJ. Synthetic biology: applications come of age. Nat Rev Genetics 2010; 11:367 - 379
- Ingram LO, Conway T, Clark DP, Sewell GW, Preston JF. Genetic engineering of ethanol production in Escherichia coli. Appl Environ Microbiol 1987; 53:2420 - 2425
- Li R, Zhang H, Qi Q. The production of polyhydroxyalkanoates in recombinant Escherichia coli. Bioresour Technol 2007; 98:2313 - 2320
- Dewick PM. The biosynthesis of C5-25 terpenoid compounds. Nat Prod Rep 2002; 19:181 - 222
- Christianson DW. Unearthing the roots of the terpenome. Curr Opin chem Biol 2008; 12:141 - 150
- Renninger NS, McPhee DJ. Fuel compositions including farnesane and farnesene derivatives and methods of making and using same US Patent 7,399,323
- Renninger NS, Ryder JA, Fisher KJ. Jetfuel compositions and methods of making and using same WO2008130492
- Song L. A soluble form of phosphatase in Saccharomyces cerevisiae capable of converting farnesyl diphosphate into E,E-farnesol. Appl Biochem Biotechnol 2006; 128:149 - 158
- Zhang YM, Rock CO. Membrane lipid homeostasis in bacteria. Nat Rev Microbiol 2008; 6:222 - 233
- Steen EJ, Kang Y, Bokinsky G, Hu Z, Schirmer A, McClure A, et al. Microbial production of fatty-acid-derived fuels and chemicals from plant biomass. Nature 2010; 463:559 - 563
- Jiang P, Cornan JE Jr. Inhibition of fatty acid synthesis in Escherichia coli in the absence of phospholipid synthesis and release of inhibition by thioesterase action. J Bacterial 1994; 176:2814 - 2821
- Cho H, Cronan JE Jr. Defective export of a periplasmic enzyme disrupts regulation of fatty acid synthesis. J Biol Chem 1995; 270:4216 - 4219
- Dennis M, Kolattukudy PE. A cobalt-porphyrin enzyme converts a fatty aldehyde to a hydrocarbon and CO. Proc Natl Acad Sci USA 1992; 82:5306 - 5310
- Wackett LP, Frias JA, Seffernick JL, Sukovich DJ, Cameron SM. Genomic and biochemical studies demonstrating the absence of an alkane-producing phenotype in Vibrio furnissii M1. Appl Environ Microbiol 2007; 73:7192 - 7198
- Friedman L, Da Costa B. Hydrocarbon-producing genes and methods of their use WO20081487781
- Rowland O, Zheng H, Hepworth SR, Lam P, Jetter R, Kunst L. CER4 encodes an alcohol-forming fatty acyl-coenzyme A reductase involved in cuticular wax production in Arabidopsis. Plant Physiol 2006; 142:866 - 877
- Doan TT, Carlsson AS, Hamberg M, Bulow L, Stymne S, Olsson P. Functional expression of five Arabidopsis fatty acyl-CoA reductase genes in Escherichia coli. J Plant Physiol 2009; 166:787 - 796
- Magnuson K, Jackowski S, Rock CO, Cronan JE Jr. Regulation of fatty acid biosynthesis in Escherichia coli. Microbiol Rev 1993; 57:522 - 542
- Whitehead TR, Hespell RB. The genes for three xylan-degrading activities from Bacteroides ovatus are clustered in a 3.8-kilobase region. J Bacteriol 1990; 172:2408 - 2412
- Adelsberger H, Hertel C, Glawischnig E, Zverlov VV, Schwarz WH. Enzyme system of Clostrium stercorarium for hydrolysis of arabinoxylan: reconstitution of the in vivo system from recombinant synzmes. Microbiology 2004; 150:2257 - 2266
- Sheehan J, Dunahay T, Benemann J, Roessler PA. A look back at the US Department of Energy's Aquatic Species Program: Biodiesel from Algae. US Department of Energy Office of Fuels Development: Closeout Report 1998; TP-580-241-24190
- Zhu X-G, Long SP, Ort DR. What is the maximum efficiency with which photosynthesis can convert solar energy into biomass?. Curr Opin Biotechnol 2008; 19:153 - 159
- Weyer KM, Bush DR, Darzins A, Wilson BD. Theoretical maximum algal oil production. Bioenerg Res 2010; 3:204 - 213
- National Algal Biofuels Technology Roadmap 2009; U.S. Department of Energy Biomass program https://e-center.doe.gov/iips/faoopor.nsf/UNID/____79E3ABCACC9AC14A852575CA00799D99/$file/AlgalBiofuels_Roadmap_7.pdf
- Maron W, Wilcox S. Solar radiation data manual for flat-plate and concentrating colle26. ctors, National Renewable Energy Laboratory (based on the National Solar Radiation Data Base (NSRDB) Version 1.1 1994;
- Gueymard C. Simple model of the atmospheric radiative transfer of sunshine (SMARTS), v 2.9.5 2005; Solar Consulting Services www.nrel.gov/rredx/smarts
- Blankenship RE. Molecular Mechanisms of Photosynthesis. Blackwell Science 2002;