Abstract
Recent recommendations by the National Science Advisory Board for Biosecurity (NSABB) to redact key methodological details of two studies involving mammal-to-mammal transmission of the H5N1 (H5) subtype influenza viruses, has led to a temporary moratorium on all research involving live H5N1 or H5 HA reassortant viruses shown to be transmissible in ferrets. Herein, I review the events which led to this impasse and comment on their impact.
History Repeating Itself
In the summer of 1974, in response to serious concerns about the safety of the then nascent field of recombinant DNA technology,Citation1 Paul Berg, chairman of the US National Academy of Science’s Committee on Recombinant DNA Molecules Assembly of Life Sciences, submitted a letter to ScienceCitation2 calling for a voluntary moratorium on certain recombinant DNA experiments which the committee deemed to be potentially dangerous. The now infamous Berg Letter also called for the establishment of “an international meeting of involved scientists from all over the world…to further discuss appropriate ways to deal with the potential biohazards of recombinant DNA molecules.”
The meeting, held the following February, 1975, at the Asilomar Conference Centre, California, marked an important milestone in the history of modern molecular biology.Citation3 On the final day of the conference, participants (which included lawyers and assorted members of the media as well as the scientists) agreed that the moratorium should be lifted, and the research allowed to continue, albeit under stringent restrictions.Citation4 The recommendations resulting from Asilomar, issued in July 1976, proved remarkably effective; informing the official US guidelines on research on recombinant DNA technology to this day.
Four year after this landmark meeting, when the dust had finally settled on the recombinant DNA controversy, Donald S. Fredrickson, the then director of the National Institutes of Health (NIH), published a history of the guidelines; concluding with the following prophetic statement:
“Faced with real questions of theoretical risks, the scientists paused and then decided to proceed with caution…..Uncertainty of risk, however, is a compelling reason for caution. It will come again in some areas of scientific research, and the initial response must be the same. After that, the lessons learned here should help us through the turbulence that is sure to come.”
Fredrickson was right, it has come again and yet again we find ourselves caught up in the turbulence surrounding so called dual-use research of concern (DURC)–that which has the potential to cause harm as well as good.Citation5,Citation6 This time the controversy is focused on the laboratory adaptation of avian H5N1 (H5) subtype influenza virus. Specifically, studies enabling the virus to move from mammal-to-mammal by respiratory transmission, and subsequent recommendations by the National Science Advisory Board for Biosecurity (NSABB–a Governmental advisory body set up to inform the research community about research involving agents that pose potential threats to national security and/or public health) to redact key manuscript details in an effort to prevent the studies from being easily replicated by individuals of “nefarious intent”Citation7.
The Story So Far
In October 2011, the NSABB was asked to review two papers–one by Ron Fouchier and colleagues of the Erasmus Medical Center in Rotterdam, the other by Yoshihiro Kawaoka and his team at the University of Wisconsin (both currently under review by Science and Nature respectively). The concerns arose from the DURC potential of the reported studies, specifically; the adaptation of the highly pathogenic avian influenza A/H5N1 virus () to infect mammalian hosts (ferrets), such that it could potentially be transmitted via respiratory droplets (or aerosols). Despite regular human contact with animal reservoirs (poultry) for at least 50 y, H5 strains which cause sustained human disease have, as yet, failed to emerge. However, notwithstanding, the NSABB felt that the mutations created by Fouchier and Kawaoka may expedite such a zoonotic shift – leading to a virus that is more easily transmissible between humans; and a potentially catastrophic pandemic. While NSABB recommended that the general conclusions of both papers be published, the manuscripts should not report the methodological detail necessary to replicate the reported experiments (NIH Press Release, http://www.nih.gov/news/health/dec2011/od-20.htm). The US Government, accepting the NSABB recommendations, relayed the imprimatur of redacted publication to the researchers and the associated scientific journals. Both parties reluctantly agreed, with the proviso that the Government devise an equitable process for sharing the details of the experiments with “responsible” scientists and clinicians.
Figure 1. Electron micrograph of the H5N1 Influenza virus. Reproduced with permission from Nature Publishing Group.
However, while the compromise went too far for many, it simply was not enough for others–a feeling crystallized by a January 8th, 2012, editorial in The New York Times entitled “An Engineered Doomsday”–calling for the newly constructed H5N1 constructs to be destroyed. Against this background of heightened public interest and fear, Fouchier (the lead author of the Science paper) and Kawaoka (of the Nature paper), along with 37 other prominent influenza researchers, published a letter in both NatureCitation8 and ScienceCitation9 calling for a voluntary 60 d moratorium on all experiments involving “live H5N1 or H5 HA reassortant viruses already shown to be transmissible in ferrets.” This latter day Berg Letter also called for an Asilomar like meeting in which “the scientific community comes together to discuss and debate these issues.” The World Health Organization convened this meeting in Geneva on 16–17th February, 2012. Attended by the lead researchers of the two offending studies, representatives of Science and Nature, bioethicists and directors from several WHO collaborating-center laboratories specializing in influenza, the Geneva symposium reached consensus on two important issues:
The temporary moratorium on research with newly modified H5N1 viruses must continue, while allowing research on naturally-occurring H5N1 influenza virus to proceed.
Delayed publication of the entire manuscript, would have more public health benefit than urgently publishing the incomplete story.
“More Sinn’d Against than Sinning”
This is not the first time the influenza community has faced the DURC issue. In 2005 a similar controversy erupted around the recreation of the infamous ‘Spanish flu’; the 1918 pandemic virus which claimed the lives of an estimated 50–100 million peopleCitation10–approximately twice the number killed during the previous four years of World War 1. Following the discovery in the 1990s of partially degraded samples of the 1918 virus, in the lung tissue of US soldiers who had succumbed to the ‘Spanish flu’, researchers were able to salvage and amplify the viral RNA.Citation11,Citation12 With the 1918 influenza virus coding sequence to hand, Tumpey and colleaguesCitation13 used a reverse genetics approach i.e. taking an existing contemporary influenza virus of lesser virulence and, one by one, swapping its genes with those from the 1918 pandemic strain – thereby creating (or recreating) a live version of the extinct ‘Spanish flu’Citation14. While the NSABB was also convened in this instance, their recommendations were far less punitive than those handed down to Fouchier and Kawaoka–the Tumpey paperCitation13 was published in full. Indeed, several other high profile influenza related studies have apparently flown below the NSABBs radar, many of which arguably pose a similar if not greater threat than that of the Fouchier and Kawaoka studies. In 2006, for example, both ScienceCitation15 and NatureCitation16 carried reports of specific mutations enabling the H5 viral haemagglutinin to bind human as opposed to avian tissues. In January of this year, a report by Chen et al.,Citation17 published in full in the journal Virology, bears an uncanny resemblance to the works of Fouchier and Kawaoka; describing mutations in an H5N1 virus which confer airborne transmissibility between ferrets. Furthermore, several recent studies have been published which describe mutations that enable transmission of other potentially pandemic strains between ferretsCitation18,Citation19 or that increase the virulence potential of currently circulating virus strains.Citation20
Echoing Fredrickson’s post-Asilomar assertion that we pause and proceed with caution, let us consider for a moment where the true risks lie. At the heart of the controversy is the assertion that the case fatality rate for human H5 infections is in the range of 50–80%.Citation21 Derived from a list of H5 cases confirmed under WHO guidelines; the list tallies 573 cases in 15 countries with an ~60% mortality rate.Citation21 However, a literature searchCitation22-Citation31 reveals seropositivity in humans resulting from H5 infections to be in the order of 0.2–5.6%, at least an order of magnitude lower than that reported by the WHO. Thus, it is entirely likely that the case fatality rate for H5N1 in humans is significantly lower than previously reported. Moreover, ferrets are not humans; as a model organism they are more susceptible to infection with influenza viruses and are also more likely than humans to exhibit disseminated, multiorgan disease including neurologic sequelae resulting from viral replication in the brain.Citation32-Citation35 Furthermore, passage of the virus in ferrets, the approach taken by Fouchier and Kawaoka, is more likely to tailor viral pathogenesis specifically to that host rather than human. Indeed, viral passage in a non-human host has frequently been used to reduce viral virulence in humans, and has been successfully applied to the generation of several attenuated viral vaccines, including poliovirus.Citation36 Rather than engineering a hypervirulent H5N1 variant for humans, the Fouchier and Kawaoka studies may in fact have led to an attenuated human variant–though this is simply unknowable from the available data. In any case, viable vaccines candidates for H5 viruses do existCitation37,Citation38 and available influenza medications have been shown to be effective against H5 strains.Citation39
Publish or Perish
Thus, perhaps our fears should focus more on the consequences of forced scientific censure, rather than the unlikely worst case scenarios (be they real or imagined) resulting from a full disclosure of the facts. Playing devil’s advocate, let us consider for a moment a scenario in which the NSABB had blocked, or redacted, publication of the Tumpey 1918 influenza paperCitation13; would the world be a safer place?
The answer is quite simply no! In this scenario, we would not know that the 1918 virus is sensitive to the seasonal flu vaccine as well as to the common flu drugs amantadine (Symmetrel) and oseltamivir (Tamiflu), and so poses no serious pandemic threat at this time.Citation39 Without the Tumpey paper,Citation13 we would still be living in fear of “nefarious intent” and the potential for ‘Spanish flu’ to be used by bioterrorists (). Blocking publication of the Fouchier and Kawaoka studies does not make the world a safer place–just a less enlightened one.
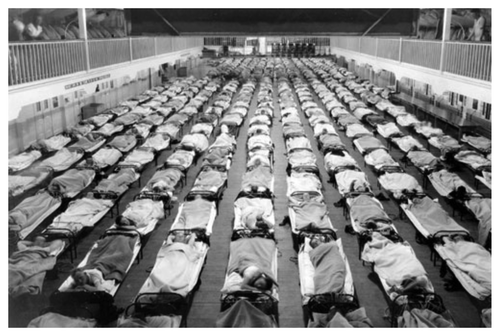
Figure 2. Scenes like the one above, from the Naval Training Station, San Francisco, California, depicting row on row of cots filled with infected patients, were common during the 1918 influenza pandemic. History must not be permitted to repeat itself, either through ignorance or neglect. Photo credit: U.S. Naval Center.
Acknowledgments
RDS is a European Society of Clinical Microbiology and Infectious Diseases Research Fellow.
References
- Singer M, Soll D. Guidelines for DNA hybrid molecules. Science 1973; 181:1114; http://dx.doi.org/10.1126/science.181.4105.1114; PMID: 11663279
- Berg P, Baltimore D, Boyer HW, Cohen SN, Davis RW, Hogness DS, et al. Letter: Potential biohazards of recombinant DNA molecules. Science 1974; 185:303; http://dx.doi.org/10.1126/science.185.4148.303; PMID: 4600381
- Whelan WJ. Asilomar: 20 years on. FASEB J 1995; 9:295; PMID: 7895999
- Berg P. Meetings that changed the world: Asilomar 1975: DNA modification secured. Nature 2008; 455:290 - 1; http://dx.doi.org/10.1038/455290a; PMID: 18800118
- Kuhlau F, Eriksson S, Evers K, Höglund AT. Taking due care: moral obligations in dual use research. Bioethics 2008; 22:477 - 87; http://dx.doi.org/10.1111/j.1467-8519.2008.00695.x; PMID: 18959730
- Zmorzynska A, Suk JE, Biederbick W, Maidhof H, Sasse J, Semenza JC, et al. Unfinished business: efforts to define dual-use research of bioterrorism concern. Biosecur Bioterror 2011; 9:372 - 8; http://dx.doi.org/10.1089/bsp.2011.0021; PMID: 22060036
- Keim PS. The NSABB Recommendations: Rationale, Impact, and Implications. MBio 2012; 3; In press http://dx.doi.org/10.1128/mBio.00021-12; PMID: 22294677
- Fouchier RA, García-Sastre A, Kawaoka Y. Pause on avian flu transmission studies. Nature 2012; 481:443; http://dx.doi.org/10.1038/481443a; PMID: 22266939
- Fouchier RA, García-Sastre A, Kawaoka Y, Barclay WS, Bouvier NM, Brown IH, et al. Pause on avian flu transmission research. Science 2012; 335:400 - 1; http://dx.doi.org/10.1126/science.1219412; PMID: 22282787
- Morens DM, Taubenberger JK, Harvey HA, Memoli MJ. The 1918 influenza pandemic: lessons for 2009 and the future. Crit Care Med 2010; 38:Suppl e10 - 20; http://dx.doi.org/10.1097/CCM.0b013e3181ceb25b; PMID: 20048675
- Taubenberger JK, Reid AH, Krafft AE, Bijwaard KE, Fanning TG. Initial genetic characterization of the 1918 “Spanish” influenza virus. Science 1997; 275:1793 - 6; http://dx.doi.org/10.1126/science.275.5307.1793; PMID: 9065404
- Taubenberger JK, Reid AH, Fanning TG. The 1918 influenza virus: A killer comes into view. Virology 2000; 274:241 - 5; http://dx.doi.org/10.1006/viro.2000.0495; PMID: 10964767
- Tumpey TM, Basler CF, Aguilar PV, Zeng H, Solórzano A, Swayne DE, et al. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science 2005; 310:77 - 80; http://dx.doi.org/10.1126/science.1119392; PMID: 16210530
- Tumpey TM, Belser JA. Resurrected pandemic influenza viruses. Annu Rev Microbiol 2009; 63:79 - 98; http://dx.doi.org/10.1146/annurev.micro.091208.073359; PMID: 19385726
- Stevens J, Blixt O, Tumpey TM, Taubenberger JK, Paulson JC, Wilson IA. Structure and receptor specificity of the hemagglutinin from an H5N1 influenza virus. Science 2006; 312:404 - 10; http://dx.doi.org/10.1126/science.1124513; PMID: 16543414
- Yamada S, Suzuki Y, Suzuki T, Le MQ, Nidom CA, Sakai-Tagawa Y, et al. Haemagglutinin mutations responsible for the binding of H5N1 influenza A viruses to human-type receptors. Nature 2006; 444:378 - 82; http://dx.doi.org/10.1038/nature05264; PMID: 17108965
- Chen LM, Blixt O, Stevens J, Lipatov AS, Davis CT, Collins BE, et al. In vitro evolution of H5N1 avian influenza virus toward human-type receptor specificity. Virology 2012; 422:105 - 13; http://dx.doi.org/10.1016/j.virol.2011.10.006; PMID: 22056389
- Kimble JB, Sorrell E, Shao HX, Martin PL, Perez DR. Compatibility of H9N2 avian influenza surface genes and 2009 pandemic H1N1 internal genes for transmission in the ferret model. Proc Natl Acad Sci U S A 2011; 108:12084 - 8; http://dx.doi.org/10.1073/pnas.1108058108; PMID: 21730147
- Pappas C, Viswanathan K, Chandrasekaran A, Raman R, Katz JM, Sasisekharan R, et al. Receptor specificity and transmission of H2N2 subtype viruses isolated from the pandemic of 1957. PLoS One 2010; 5:e11158; http://dx.doi.org/10.1371/journal.pone.0011158; PMID: 20574518
- Houng HS, Garner J, Zhou Y, Lyons A, Kuschner R, Deye G, et al. Emergent 2009 influenza A(H1N1) viruses containing HA D222N mutation associated with severe clinical outcomes in the Americas. J Clin Virol 2012; 53:12 - 5; http://dx.doi.org/10.1016/j.jcv.2011.09.004; PMID: 22036040
- Palese P, Wang TT. H5N1 influenza viruses: Facts, not fear. Proc Natl Acad Sci U S A 2012; 109:2211 - 3; http://dx.doi.org/10.1073/pnas.1121297109; PMID: 22308474
- Bridges CB, Lim W, Hu-Primmer J, Sims L, Fukuda K, Mak KH, et al. Risk of influenza A (H5N1) infection among poultry workers, Hong Kong, 1997-1998. J Infect Dis 2002; 185:1005 - 10; http://dx.doi.org/10.1086/340044; PMID: 11930308
- Buchy P, Vong S, Chu S, Garcia JM, Hien TT, Hien VM, et al. Kinetics of neutralizing antibodies in patients naturally infected by H5N1 virus. PLoS One 2010; 5:e10864; http://dx.doi.org/10.1371/journal.pone.0010864; PMID: 20532246
- Cavailler P, Chu S, Ly S, Garcia JM, Ha Q, Bergeri I, et al. Seroprevalence of anti-H5 antibody in rural Cambodia, 2007. J Clin Virol 2010; 48:123 - 6; http://dx.doi.org/10.1016/j.jcv.2010.02.021; PMID: 20356781
- Dejpichai R, Laosiritaworn Y, Phuthavathana P, Uyeki TM, O’Reilly M, Yampikulsakul N, et al. Seroprevalence of antibodies to avian influenza virus A (H5N1) among residents of villages with human cases, Thailand, 2005. Emerg Infect Dis 2009; 15:756 - 60; http://dx.doi.org/10.3201/eid1505.080316; PMID: 19402962
- Santhia K, Ramy A, Jayaningsih P, Samaan G, Putra AA, Dibia N, et al. Avian influenza A H5N1 infections in Bali Province, Indonesia: a behavioral, virological and seroepidemiological study. Influenza Other Respi Viruses 2009; 3:81 - 9; http://dx.doi.org/10.1111/j.1750-2659.2009.00069.x; PMID: 19459276
- Schultsz C, Nguyen VD, Hai T, Do QH, Peiris JS, Lim W, et al. Prevalence of antibodies against avian influenza A (H5N1) virus among Cullers and poultry workers in Ho Chi Minh City, 2005. PLoS One 2009; 4:e7948; http://dx.doi.org/10.1371/journal.pone.0007948; PMID: 19956765
- Vong S, Ly S, Van Kerkhove MD, Achenbach J, Holl D, Buchy P, et al. Risk factors associated with subclinical human infection with avian influenza A (H5N1) virus--Cambodia, 2006. J Infect Dis 2009; 199:1744 - 52; http://dx.doi.org/10.1086/599208; PMID: 19416078
- Khuntirat BP, Yoon IK, Blair PJ, Krueger WS, Chittaganpitch M, Putnam SD, et al. Evidence for subclinical avian influenza virus infections among rural Thai villagers. Clin Infect Dis 2011; 53:e107 - 16; http://dx.doi.org/10.1093/cid/cir525; PMID: 21921216
- Lu CY, Lu JH, Chen WQ, Jiang LF, Tan BY, Ling WH, et al. Potential infections of H5N1 and H9N2 avian influenza do exist in Guangdong populations of China. Chin Med J (Engl) 2008; 121:2050 - 3; PMID: 19080274
- Wang M, Fu CX, Zheng BJ. Antibodies against H5 and H9 avian influenza among poultry workers in China. N Engl J Med 2009; 360:2583 - 4; http://dx.doi.org/10.1056/NEJMc0900358; PMID: 19516044
- Zitzow LA, Rowe T, Morken T, Shieh WJ, Zaki S, Katz JM. Pathogenesis of avian influenza A (H5N1) viruses in ferrets. J Virol 2002; 76:4420 - 9; http://dx.doi.org/10.1128/JVI.76.9.4420-4429.2002; PMID: 11932409
- Lednicky JA, Hamilton SB, Tuttle RS, Sosna WA, Daniels DE, Swayne DE. Ferrets develop fatal influenza after inhaling small particle aerosols of highly pathogenic avian influenza virus A/Vietnam/1203/2004 (H5N1). Virol J 2010; 7:231; http://dx.doi.org/10.1186/1743-422X-7-231; PMID: 20843329
- Bodewes R, Rimmelzwaan GF, Osterhaus AD. Animal models for the preclinical evaluation of candidate influenza vaccines. Expert Rev Vaccines 2010; 9:59 - 72; http://dx.doi.org/10.1586/erv.09.148; PMID: 20021306
- Yen HL, Lipatov AS, Ilyushina NA, Govorkova EA, Franks J, Yilmaz N, et al. Inefficient transmission of H5N1 influenza viruses in a ferret contact model. J Virol 2007; 81:6890 - 8; http://dx.doi.org/10.1128/JVI.00170-07; PMID: 17459930
- Salk D, Salk J. Vaccinology of poliomyelitis. Vaccine 1984; 2:59 - 74; http://dx.doi.org/10.1016/S0264-410X(98)90035-4; PMID: 6099644
- Chen GL, Subbarao K. Live attenuated vaccines for pandemic influenza. Curr Top Microbiol Immunol 2009; 333:109 - 32; http://dx.doi.org/10.1007/978-3-540-92165-3_5; PMID: 19768402
- Galli G, Medini D, Borgogni E, Zedda L, Bardelli M, Malzone C, et al. Adjuvanted H5N1 vaccine induces early CD4+ T cell response that predicts long-term persistence of protective antibody levels. Proc Natl Acad Sci U S A 2009; 106:3877 - 82; http://dx.doi.org/10.1073/pnas.0813390106; PMID: 19237568
- Medina RA, Manicassamy B, Stertz S, Seibert CW, Hai R, Belshe RB, et al. Pandemic 2009 H1N1 vaccine protects against 1918 Spanish influenza virus. Nat Commun 2010; 1:28; http://dx.doi.org/10.1038/ncomms1026; PMID: 20975689