Abstract
Transforming acidic coiled coil 3 (TACC3) is a non-motor microtubule-associated protein (MAP) that is important for mitotic spindle stability and organisation. The exact mechanism by which TACC3 acts at microtubules to stabilise the spindle has been unclear. However, several recent studies identified that the TACC3 complex at microtubules contains clathrin in addition to its previously identified binding partner, colonic and hepatic tumour over-expressed gene (ch-TOG). In this complex, phosphorylated TACC3 interacts directly with both ch-TOG and clathrin heavy chain, promoting accumulation of all complex members at the mitotic spindle. This complex stabilises kinetochore fibres within the spindle by forming cross-bridges that link adjacent microtubules in these bundles. So, TACC3 is an adaptor that recruits ch-TOG and clathrin to mitotic microtubules, in an Aurora A kinase-regulated manner. In this mini-review we will describe the recent advances in the understanding of TACC3 function and present a model that pulls together these new data with previous observations.
Introduction
At the onset of mitosis the microtubule network becomes highly dynamic, and undergoes a large-scale reorganization from the interphase array to form the mitotic spindle.Citation1 This bipolar array of microtubules has the important job of segregating pairs of chromatids to ensure that each daughter cell receives one of each type of chromosome.Citation1 In animal cells the centrosomes nucleate microtubule assembly early in mitosis.Citation1 The less dynamic ‘minus’ ends of the microtubules are held at the centrosome while the highly dynamic ‘plus’ ends grow into the cytoplasm.Citation1 Microtubules growing out from centrosomes capture chromosomes, which then become aligned at the center of the spindle as all kinetochores become attached. Once all sisters are aligned they separate and are pulled towards the spindle poles. These complicated processes are coordinated by a vast number of proteins located at centrosomes, on the microtubules, and at the kinetochores of the chromosomes.Citation1 These include motor proteins which interact with microtubules and move along them in an ATP-dependent manner, such as kinesin 5 which contributes to spindle bipolarity and size by sliding apart overlapping microtubules originating from opposite spindle poles.Citation2 Other microtubule-associated proteins lack motor activity but play roles in spindle stability. Over recent years many such MAPs have been identified, and their individual contributions to spindle assembly and function elucidated.Citation2 One such protein is TACC3, a protein that is conserved across metazoans and down to yeast.Citation3
Early work showed that TACC3 contributes to spindle stability and organization during mitosis, by acting both at centrosomes and microtubules.Citation4,Citation5 The precise mechanism by which TACC3 exerts its spindle-stabilizing function at microtubules was unclear, but now recent evidence has shown that TACC3 exists in a complex with ch-TOG and clathrin, that cross-links microtubules in kinetochore fibers. These fibers are bundles of microtubules that link the kinetochores to the spindle poles, and are more stable than the other types of microtubule in the spindle. These bundles have the strength to withstand the forces involved in maintaining chromosome alignment and separation, and when they are disrupted, cells have difficulty accomplishing both of these events.Citation6
TACC3: A Conserved Mitotic Protein
The first hint that TACC domain-containing proteins had a role in mitosis came with the discovery of the Drosophila melanogaster protein D-TACC.Citation7 This was found to be a centrosomal protein with some localization to spindle microtubules, with an important role in spindle assembly, organization and function.Citation7 Like Drosophila, many species including Xenopus laevis and Caenhorabditis elegans contain only one TACC protein. However humans contain a family of three TACC proteins that all contain a C-terminal coiled coil domain, but vary extensively in the sequence of the N terminal part of the protein.Citation3 Of these, TACC3 most closely resembles the TACC homologs in lower species because it functions during mitosis. In humans, TACC3 is expressed in certain proliferative tissues including testis, lung, spleen, bone marrow, thymus and peripheral blood leukocytes.Citation3 This is similar in adult mice, but TACC3 is expressed in most tissue types during mouse embryo-genesis, and loss of TACC3 is lethal at the embryonic stage.Citation3 These observations are consistent with a critical role for TACC3 in cell division. This review will focus on human proteins unless specified otherwise.
TACC3 expression is altered in some human tumor types.Citation3,Citation8 However, since it is upregulated in some cancers, while being downregulated in others, it is unclear whether this event contributes to the progression of the disease. On the other hand, there is good evidence to suggest that TACC3 promotes the resistance of tumors to challenges that could otherwise cause cell death. For example, tumor cells frequently have extra centrosomes, which could potentially cause multipolar spindles and multiplanar division, leading to cell death. TACC3 and ch-TOG are involved in clustering these centrosomes into two poles, allowing a somewhat ‘normal’ division along a single axis.Citation9 Resistance of cancer cells to the spindle toxin paclitaxel also depends on TACC3, highlighting the potential clinical relevance of dysregulation of these proteins in tumors.Citation8
Unraveling the Function of TACC3
In cells, TACC3 is localized to both centrosomes and spindle microtubules, but is not found at astral microtubules once the spindle has formed.Citation5 It has a conserved function in spindle stability indicated by defects seen on depletion of the protein which include: disorganized spindles with lower microtubule density, chromosome misalignment and a longer duration of mitosis.Citation10
Homologs of TACC3 and ch-TOG have a conserved interaction,Citation3 and this had long been considered to be the functionally relevant complex. Although both proteins are found at centrosomes in human cells, their recruitment to these is independent of each other.Citation5,Citation10 Ch-TOG clearly has a critical TACC3-independent function at centrosomes where it promotes microtubule assembly and outgrowth,Citation4,Citation11,Citation12 and it also contributes to spindle bipolarity.Citation4,Citation10,Citation12 Now several recent studies have identified that TACC3 and ch-TOG on spindle microtubules are in complex with clathrin.Citation13–Citation16 These new data indicate that, at spindle fibers, TACC3 and ch-TOG function together with clathrin as part of a kinetochore fiber-stabilizing complex, rather than as a pair as previously thought.
During mitosis, clathrin has the same distribution on microtubules as TACC3 and ch-TOG.Citation13 Like these proteins, clathrin was also found to stabilize kinetochore fibers.Citation17 The observation that clathrin is only seen at microtubules during mitosis and not interphase suggests that spindle localization depends either upon a mitotic binding partner that is not available during interphase, or upon mitosis-specific modification of clathrin.Citation17,Citation18
The effect of clathrin depletion on mitosis is strikingly similar to that of TACC3 depletion.Citation10,Citation17 Indeed, the mitotic defects observed on depletion of either TACC3 or clathrin heavy chain (CHC) were no less severe than those seen on combined depletion of both proteins, confirming that they are part of the same functional complex at microtubules, which acts to stabilize kinetochore fibers.Citation16
It was already known that TACC3 is required for localization of ch-TOG to the microtubules,Citation10 but depletion of each complex member using RNA interference suggested that TACC3, CHC and ch-TOG each contribute somewhat to the localization of the other complex members to the spindle.Citation10,Citation13 Depletion of TACC3, and to a lesser extent depletion of ch-TOG, caused a reduction in the amount of clathrin at the spindle.Citation13 Interestingly, CHC depletion using RNA interference resulted in a reduction in the amount of TACC3 at the spindle, even when the loss in microtubule density that occurs was accounted for.Citation13–Citation16 Depletion of CHC caused a loss of ch-TOG from microtubules,Citation13,Citation16 much like TACC3 depletion.Citation10,Citation13,Citation16 From these data it seems that all three proteins help the others to become enriched at the spindle.
When GFP-TACC3 was overexpressed in cells, it recruited additional clathrin and ch-TOG to the spindle, while over-expression of CHC and ch-TOG had no effect on the other two.Citation11,Citation13 This suggests that TACC3 is the limiting factor that recruits the complex, but that the complex as a whole interacts more stably with the spindle than any individual components.Citation13 This marks a distinction between recruitment and enrichment of complex members at spindles.
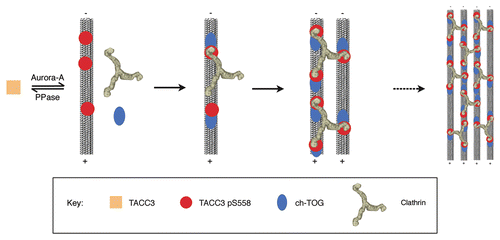
Co-immunoprecipitation of CHC and TACC3 was dependent on Aurora A kinase activity,Citation14,Citation16 and a direct interaction was found between TACC3 and CHC that is dependent upon phosphorylation of TACC3 by Aurora A kinase, at serine residue 558 and possibly serine 552.Citation14,Citation16 This phosphorylation event appears to be regulated by the Ran GTPase via Importin β.Citation14 Treatment of cells with an Aurora A kinase inhibitor resulted in loss of TACC3 and clathrin from microtubules,Citation13,Citation19 suggesting that phosphorylation by Aurora A both facilitates initial recruitment of TACC3 to microtubules, and creates a binding site to facilitate recruitment of CHC.
Thus, there are two aspects to localization of this complex to microtubules; firstly TACC3-dependent recruitment to microtubules, and secondly the subsequent accumulation of the whole complex here.Citation13 In this model the phosphorylation of TACC3 could cause a conformational change to permit microtubule binding, similar to the change reported for the Xenopus TACC3 homolog Maskin.Citation20 In this way phosphorylation would contribute to initial recruitment. Accumulation would occur as the proteins interact with each other in a large complex that forms multiple contacts with microtubules. Phosphorylation would also contribute to this accumulation since the interaction of CHC with TACC3 is also phosphodependent.Citation14,Citation16 Further work is necessary to understand the fine detail of how the components are recruited and accumulated at spindle fibers. It is clear that the TACC3/ch-TOG/clathrin complex is required for stabilization of kinetochore fibers, so how does the complex do it?
Pulling It Together: The TACC3/ch-TOG/Clathrin Complex is an Inter-Microtubule Bridge in Kinetochore Fibers
We are beginning to understand how kinetochore fibers can be stabilized. It had been noted for many years that in electron micrographs, electron-dense bridges appear to link adjacent microtubules within these fibers, raising the possibility that these bridges act to stabilize the fibers.Citation21 It is already appreciated that some proteins form bridges between antiparallel microtubules at the midzone of the spindle where the microtubules nucleated from the opposite poles overlap.Citation2 But little was known about the proteins which bridge between parallel microtubules in the spindle.
Clathrin exists as a triskelion formed by association of three CHCs via their C termini, with each CHC forming one of the legs, each of which is tightly associated with a clathrin light chain (CLC).Citation22 The globular N-terminal domain or ‘foot’ is located at the end of each CHC leg. This foot domain is needed for localization of clathrin to the spindle.Citation17,Citation23 This together with the kinetochore fiber stabilizing function of clathrin raised the intriguing possibility that clathrin may be an important component of one such inter-microtubule bridge, in which it could link adjacent microtubules via its connected ‘feet’.Citation13,Citation17 Interestingly, a CHC gene fusion that forms dimers rather than trimers that was identified in human tumors can rescue the mitotic function of clathrin in cells depleted of CHC,Citation24 while mutants that remain monomeric cannot.Citation23 Thus, multimerization of CHCs is essential, but it does not have to be a trimer to accomplish kinetochore fiber stabilization.
There is now ultrastructural evidence to support this idea of clathrin as an inter-microtubule bridge. When CHC was depleted there were fewer bridges within kinetochore fibers, and these microtubule bundles contained fewer total microtubules, and a lower density of microtubules.Citation13 Immunogold labelling with an anti-clathrin antibody revealed that gold particles were frequently found in between pairs of closely located microtubules, and were often seen at inter-microtubule bridges, confirming clathrin as a bridge component.Citation13 Furthermore, the bridges that were lost on CHC depletion were a specific subtype of short-length bridges.Citation13
TACC3 depletion also caused loss of the same class of short bridges.Citation13 Together this suggests that, regardless of the order of recruitment, the mode of action of this TACC3/ch-TOG/clathrin complex is to stabilize kinetochore fibers by physically bridging between adjacent microtubules. This model is represented in . In addition to physical cross-bracing of microtubules that make up the spindle, the TACC3/ch-TOG/clathrin bridges also appear to protect the fiber from microtubule loss.Citation13 This could occur as a consequence of the cross-bracing activity and/or via the activity of ch-TOG.Citation13,Citation16 It is known that in vitro the Xenopus ch-TOG homolog, XMAP215, acts as a processive tubulin polymerase, employing both a microtubule binding site and a variable number of tubulin dimer binding sites to incorporate new dimers into the end of a microtubule.Citation25 In cells, ch-TOG promotes growth of microtubule plus ends by antagonizing mitotic centromere-associated kinesin (MCAK).Citation12 MCAK promotes phases of microtubule depolymerization, known as ‘catastrophes’. This anti-catastrophe activity of ch-TOG may be mediated by its polymerase activity balancing MCAK activity, or possibly by physically protecting plus ends against MCAK.Citation12 As some groups suggest, there could therefore be an additional aspect to the stabilizing function of the TACC3/ch-TOG/clathrin complex, perhaps by co-ordinating or increasing the ch-TOG anti-catastrophe activity down the microtubules.Citation13,Citation16 Thus, the role of TACC3 in mitosis is as a regulated adaptor that can integrate several mitotic proteins into a complex on micro-tubules which holds kinetochore fibers together.
Remaining Questions
Work published over the past year has accelerated our understanding of TACC3, ch-TOG and clathrin function at the mitotic spindle.Citation13–Citation16 However there are, as always, some unresolved questions. Clearly this complex is found at kinetochore microtubules, but whether it is also found at interpolar microtubules, and if not, how specificity for kinetochore micro-tubules would be achieved is not known.
It is also not clear how TACC3 interacts with microtubules. Maskin is able to interact directly with microtubules,Citation26 but the human protein does not appear to do so.Citation16 In cells, recruitment of both TACC3 and Maskin to centrosomes and spindles requires phosphorylation by Aurora A kinase.Citation3 Together this led some groups to propose that clathrin is actually the recruitment factor for TACC3, and by extension ch-TOG, to the spindle.Citation14–Citation16 In this scheme it is not clear how clathrin would be recruited to microtubules. Indeed since CHC does not interact directly with microtubules and in cells only a small proportion of clathrin is found at the spindle, while the rest is found in the cytoplasm and at clathrin coated structures on membranes, clathrin is a very unlikely candidate as a spindle recruitment factor.Citation13 Interestingly, the part of CHC that binds to phospho-TACC3 includes part of CHC repeat 0 that was identified previously as critical for recruitment of CHC to the spindle.Citation16,Citation18 This is consistent with the unified model presented here (), since if clathrin itself were the spindle recruitment factor, it would not need to be able to bind TACC3 in order to become localized to the spindle.
There are also some outstanding questions about the bridging function of this complex and the function of TACC3 at the spindle. While the trimeric structure of clathrin makes it an attractive candidate as the central bridging portion of the complex, TACC3 is also known to dimerize,Citation27 raising the possibility of an alternative conformation in which dimerized TACC3 could bridge between adjacent microtubules by interacting with a clathrin molecule on each microtubule. Interestingly it has been shown in fission yeast that the TACC3 homolog, Alp7, cross-links both antiparallel and parallel microtubules.Citation28
We are lacking complete structural information for all complex components. A structure has been determined for the single TOG domain from the C. elegans ch-TOG homolog, Zyg9, however the structure of the whole human ch-TOG, particularly when bound to microtubules, is unknown.Citation29 An atomic model for clathrin has been described,Citation22 but there is currently no structural information for TACC3. The configuration of bridges shown in takes into account the known spacing of clathrin triskelia and microtubules.Citation13 Future structural studies may well refine this model. Furthermore, if clathrin is indeed the central bridging portion of the complex, as has been suggested,Citation13 then does it exist as a single triskelion? CHC mutants that are predicted to be unable to form lattices can function in mitosis,Citation23,Citation24 but whether bridges are interconnected needs to be explored further.
One study suggested that G2 and S-phase expressed 1 (GTSE1) may also be part of the TACC3/ch-TOG/clathrin complex.Citation15 Whether GTSE1 is an important part of this bridging complex is unclear at this stage. It will be interesting to see whether loss of GTSE1 affects inter-microtubule bridges.
It also remains unclear to what extent TACC3 functions at centrosomes. TACC3 has been reported to localize to centrosomes, but it is not required for ch-TOG recruitment here, suggesting that these proteins may not interact at centrosomes in human cells.Citation5,Citation10 Clathrin has no prominent localization at centrosomes.Citation13,Citation17–Citation19,Citation23,Citation24 Certainly, ch-TOG acts independently of TACC3 at centrosomes to promote microtubule assembly.Citation4 Taken together this suggests that ch-TOG functions alone at centrosomes, although the possibility that the other TACC proteins could be involved cannot be excluded.Citation10 Once short microtubules are generated from the centrosomes by ch-TOG, TACC3 seems to aid their stability,Citation4,Citation11,Citation12 which we predict is as part of the TACC3/ch-TOG/clathrin complex described in this review.
While the TACC3/ch-TOG interaction is conserved across many species, currently it has only been shown in humans and Xenopus that clathrin is also contained within this complex.Citation13–Citation16 Further work is needed to determine whether this is conserved, or if the function of these proteins may be clathrin-independent in some species. In particular, in some species it seems that TACC3 homologs are more involved in centrosome-based function, such as in Drosophila, where D-TACC is required for localization of the ch-TOG homolog, Msps, to centrosomes.Citation30
Studying this complex is not without complications. Clathrin has a fundamentally important role during interphase, when clathrin mediated membrane traffic is critical to many cellular functions, making knockout mice or cell lines an unrealistic prospect.Citation22 TACC3 knockout is also lethal before birth.Citation3 With assembly and organization of the mitotic spindle being so tightly regulated, it is difficult to tease out the functions of any critical spindle protein by RNA interference, since any defects occurring early on in the process may mask defects at later stages. This is a challenge in cell cycle research generally, but newer methods of acute inhibition or inactivation of a protein will enable us to analyse effects on a shorter time scale.
Summary
In this short review, we have drawn together recent work on the function of TACC3 in mitosis. We have seen that TACC3 forms a complex that pulls together microtubules. These cross-linking bridges help to stabilize the kinetochore fibers that control chromosome dynamics. In this role, TACC3 seems to act as a regulated adaptor, recruiting other proteins to the microtubules once it is phosphorylated by Aurora A kinase. Once this initial recruitment is achieved, formation of multiple bridges may facilitate accumulation of all complex members at microtubules. The TACC3/ch-TOG/clathrin bridges that we have discussed represent a subset of inter-microtubule bridges. The identification of the other inter-microtubule bridges is an exciting prospect indeed for those interested in the function of the mitotic spindle.
Abbreviations
| CHC | = | clathrin heavy chain |
| CLC | = | clathrin light chain |
| TACC3 | = | transforming acidic coiled coil 3 |
| ch-TOG | = | colonic and hepatic tumor overexpressed gene |
| XMAP215 | = | Xenopus microtubule associated protein 215 kDa |
| Msps | = | minispindles |
| Zyg9 | = | ZYGote defective: embryonic lethal family member 9 |
| MAP | = | microtubule associated protein |
| MCAK | = | mitotic centromere-associated kinesin |
| GTSE1 | = | G2 and S-phase expressed 1 |
Figures and Tables
Figure 1 Model of recruitment and inter-microtubule cross-linking by the TACC3/ch-TOG/clathrin complex. Phosphorylation of TACC3 by Aurora A kinase enables it to bind to microtubules, where it can recruit clathrin and ch-TOG. Clathrin triskelia could interact with multiple TACC3 molecules on microtubules, including those on adjacent microtubules, in which case a bridge would be formed. Ultimately many bridges would form between closely located microtubules, helping to stabilize microtubule bundles in kinetochore fibers. As the multiple contacts form in these complexes, all the individual components would interact more stably with the spindle, causing the accumulation we observe by immunofluorescence.
Acknowledgments
We would like to thank members of the Royle lab for useful discussion and we apologize to those whose work we could not discuss due to space constraints. Our work is supported by a Career establishment Award from Cancer Research UK (C25425/A8722).
References
- O'Connell CB, Khodjakov AL. Cooperative mechanisms of mitotic spindle formation. J Cell Sci 2007; 120:1717 - 1722
- Peterman EJ, Scholey JM. Mitotic microtubule cross-linkers: insights from mechanistic studies. Curr Biol 2009; 19:1089 - 1094
- Peset I, Vernos I. The TACC proteins: TACC-ling microtubule dynamics and centrosome function. Trends Cell Biol 2008; 18:379 - 388
- Barr AR, Gergely F. MCAK-independent functions of ch-Tog/XMAP215 in microtubule plus-end dynamics. Mol Cell Biol 2008; 28:7199 - 7211
- Gergely F, Karlsson C, Still I, Cowell J, Kilmartin J, Raff JW. The TACC domain identifies a family of centrosomal proteins that can interact with microtubules. Proc Natl Acad Sci USA 2000; 97:14352 - 14357
- Rieder CL. Kinetochore fiber formation in animal somatic cells: dueling mechanisms come to a draw. Chromosoma 2005; 114:310 - 318
- Gergely F, Kidd D, Jeffers K, Wakefield JG, Raff JW. D-TACC: a novel centrosomal protein required for normal spindle function in the early Drosophila embryo. EMBO J 2000; 19:241 - 252
- Schmidt S, Schneider L, Essmann F, Cirstea IC, Kuck F, Kletke A, et al. The centrosomal protein TACC3 controls paclitaxel sensitivity by modulating a premature senescence program. Oncogene 2010; 29:6184 - 6192
- Fielding AB, Lim S, Montgomery K, Dobreva I, Dedhar S. A critical role of integrin-linked kinase, ch-TOG and TACC3 in centrosome clustering in cancer cells. Oncogene 2011; 30:521 - 534
- Gergely F, Draviam VM, Raff JW. The ch-TOG/ XMAP215 protein is essential for spindle pole organization in human somatic cells. Genes Dev 2003; 17:336 - 341
- Cassimeris L, Becker B, Carney B. TOGp regulates microtubule assembly and density during mitosis and contributes to chromosome directional instability. Cell Motil Cytoskeleton 2009; 66:535 - 545
- Holmfeldt P, Stenmark S, Gullberg M. Differential functional interplay of TOGp/XMAP215 and the KinI kinesin MCAK during interphase and mitosis. EMBO J 2004; 23:627 - 637
- Booth DG, Hood FE, Prior IA, Royle SJ. A TACC3/ch-TOG/clathrin complex stabilises kinetochore fibres by inter-microtubule bridging. EMBO J 2011; 30:906 - 919
- Fu W, Tao W, Zheng P, Fu J, Bian M, Jiang Q, et al. Clathrin recruits phosphorylated TACC3 to spindle poles for bipolar spindle assembly and chromosome alignment. J Cell Sci 2010; 123:3645 - 3651
- Hubner NC, Bird AW, Cox J, Splettstoesser B, Bandilla P, Poser I, et al. Quantitative proteomics combined with BAC TransgeneOmics reveals in vivo protein interactions. J Cell Biol 2010; 189:739 - 754
- Lin CH, Hu CK, Shih HM. Clathrin heavy chain mediates TACC3 targeting to mitotic spindles to ensure spindle stability. J Cell Biol 2010; 189:1097 - 1105
- Royle SJ, Bright NA, Lagnado L. Clathrin is required for the function of the mitotic spindle. Nature 2005; 434:1152 - 1157
- Hood FE, Royle SJ. Functional equivalence of the clathrin heavy chains CHC17 and CHC22 in endocytosis and mitosis. J Cell Sci 2009; 122:2185 - 2190
- Cheeseman LP, Booth DG, Hood FE, Prior IA, Royle SJ. Aurora A kinase activity is required for localisation of TACC3/ch-TOG/clathrin inter-microtubule bridges. Commun Integr Biol 2011; 4:409 - 412
- Albee AJ, Wiese C. Xenopus TACC3/maskin is not required for microtubule stability but is required for anchoring microtubules at the centrosome. Mol Biol Cell 2008; 19:3347 - 3356
- Hepler PK, McIntosh JR, Cleland S. Intermicrotubule bridges in mitotic spindle apparatus. J Cell Biol 1970; 45:438 - 444
- Royle SJ. The cellular functions of clathrin. Cell Mol Life Sci 2006; 63:1823 - 1832
- Royle SJ, Lagnado L. Trimerisation is important for the function of clathrin at the mitotic spindle. J Cell Sci 2006; 119:4071 - 4078
- Blixt MK, Royle SJ. Clathrin heavy chain gene fusions expressed in human cancers: analysis of cellular functions. Traffic 2011; 12:754 - 761
- Widlund PO, Stear JH, Pozniakovsky A, Zanic M, Reber S, Brouhard GJ, et al. XMAP215 polymerase activity is built by combining multiple tubulin-binding TOG domains and a basic lattice-binding region. Proc Natl Acad Sci USA 2011; 108:2741 - 2746
- O'Brien LL, Albee AJ, Liu L, Tao W, Dobrzyn P, Lizarraga SB, et al. The Xenopus TACC homologue, maskin, functions in mitotic spindle assembly. Mol Biol Cell 2005; 16:2836 - 2847
- Simpson RJ, Yi Lee SH, Bartle N, Sum EY, Visvader JE, Matthews JM, et al. A classic zinc finger from friend of GATA mediates an interaction with the coiled-coil of transforming acidic coiled-coil 3. J Biol Chem 2004; 279:39789 - 39797
- Thadani R, Ling YC, Oliferenko S. The fission yeast TACC protein Mia1p stabilizes microtubule arrays by length-independent crosslinking. Curr Biol 2009; 19:1861 - 1868
- Al-Bassam J, Larsen NA, Hyman AA, Harrison SC. Crystal structure of a TOG domain: conserved features of XMAP215/Dis1-family TOG domains and implications for tubulin binding. Structure 2007; 15:355 - 362
- Lee MJ, Gergely F, Jeffers K, Peak-Chew SY, Raff JW. Msps/XMAP215 interacts with the centrosomal protein D-TACC to regulate microtubule behaviour. Nat Cell Biol 2001; 3:643 - 649