Abstract
Aspects of cellular architecture, such as cytoskeletal asymmetry cues, play critical roles in directing the placement of organelles and establishing the sites of their formation. In the model green alga Chlamydomonas, the photosensory eyespot occupies a defined position in relation to the flagella and microtubule cytoskeleton. Investigations into the cellular mechanisms of eyespot placement and assembly have aided our understanding of the interplay between cytoskeletal and plastid components of the cell. The eyespot, which must be assembled anew after each cell division, is a multi-layered organelle consisting of stacks of carotenoid-filled pigment granules in the chloroplast and rhodopsin photoreceptors in the plasma membrane. Placement of the eyespot is determined on both the latitudinal and longitudinal axes of the cell by the daughter four-membered (D4) microtubule rootlet. Recent findings have contributed to the hypothesis that the eyespot photoreceptor molecules are directed from the Golgi to the daughter hemisphere of the cell and trafficked along the D4 microtubule rootlet. EYE2, a chloroplast-envelope protein, forms an elliptical patch together with the photoreceptors and establishes the site for assembly of the pigment granule arrays in the chloroplast, connecting the positioning information of the cytoskeleton to assembly of the pigment granule arrays in the chloroplast.
Introduction
Asymmetry is an essential characteristic of cytoarchitecture in both prokaryoticCitation1 and eukaryotic systems. The defined organization of organelles and other subcellular structures, such as protein complexes and microtubule networks, establishes asymmetry and polarity critical for such diverse responses to stimuli as directed cell migration,Citation2 flagellar motility,Citation3 and asymmetric division.Citation4,Citation5 In most metazoans, a conserved organelle, the centriole, transmits positional information via connections to the nucleus and microtubule cytoskeleton.Citation6 The centrioles serve as an organizing center for cytoplasmic microtubules and microtubule-based structures such as flagella and primary cilia.Citation6 The inherent polarity of cytoskeletal filamentsCitation7 allows for directional trafficking of proteins and organelles, contributing to the defined patterning of subcellular elements.
Insights into the connections between the cytoskeleton and the biogenesis and placement of organelles have come from the model unicellular green alga Chlamydomonas reinhardtii. Chlamydomonas cells have two flagella at the anterior pole, nucleated by the mother and daughter basal bodies (interphase analogs to centrioles), whereas the posterior two-thirds of the cell are filled by a single chloroplast ().Citation8,Citation9 The cytoskeleton includes four sets of highly stable microtubule bundles called rootlets which extend from the region of the basal bodies toward the posterior of the cell and possess a high degree of acetylation, a post-translational modification which augments stability.Citation10 In common with many green algae, Chlamydomonas cells perceive light levels and direction via a photosensory organelle known as the eyespot.Citation11,Citation12 Light-induced ion influxes from the eyespot region result in changes in flagellar beat patterns and swimming direction.Citation13 The eyespot itself is a complex structure consisting of stacks of carotenoid-rich globules in the chloroplast termed pigment granules, which are closely packed against thylakoid membranes and the chloroplast envelope membranes,Citation14 and an elliptical patch of light-gated photoreceptors in the plasma membrane known as channelrhodopsins (ChRs).Citation15–Citation17 The phototactic responses of the cell require the eyespot to occupy a defined, asymmetric position in the cell relative to the flagella.Citation18 In C. reinhardtii, the eyespot maintains a characteristic association with the daughter four-membered (D4) microtubule rootlet.Citation19,Citation20 As the Chlamydomonas eyespot forms anew after every cell division, it thus serves as a useful model for the study of both organelle biogenesis and cellular asymmetry. In this perspective, we review recent findings and hypotheses developed in our investigations of the propagation of asymmetry in Chlamydomonas and the mechanisms by which the eyespot is coordinately assembled from multiple cellular elements.
Propagation of Asymmetry in Chlamydomonas
The centriole plays a key role in propagating cell organization through cell division. In Chlamydomonas, the mother centriole, inherited from the previous cell cycle and acting as a basal body during interphase, possesses ultrastructural features and associated proteins not shared by the daughter basal body, distinctions presumably responsible for physiological differences between the two flagella.Citation21–Citation23 During telophase, the cleavage furrow spreads along the plane marked by the mother and daughter four-membered microtubule rootlets, bisecting the area defined by the eyespot, which, in C. reinhardtii, disintegrates prior to cell division.Citation24 The daughter basal body/rootlet assemblage inherited by one of the cells becomes the mother basal body and rootlets, while a new two-membered and four-membered microtubule rootlet begin to extend from the region of the new daughter basal body.Citation25 The new eyespot assembles near the plus-end of the nascent daughter four-membered (D4) rootlet.Citation18 The D4 rootlet itself manifests asymmetry, as it is more extensively acetylated and longer than the other rootlets during interphase.Citation26 The differences between rootlets presumably reflect the aforementioned differences between the mother and daughter basal bodies.
Asymmetric Properties of the Cytoskeleton Direct Eyespot Placement
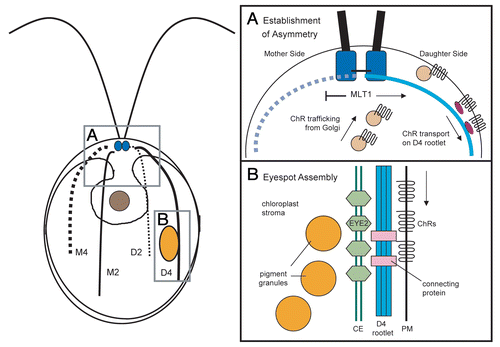
A combination of genetics and cell biology has allowed progress in understanding the mechanisms and molecular players that connect the asymmetry of the basal bodies to the establishment of the eyespot at its characteristic location. In mutants with defects in basal body assembly/segregation and aberrant cytoskeletal organization, the eyespot photoreceptor channelrhodopsin-1 (ChR1) was found to be associated with a subset of supernumerary acetylated microtubules, confirming that photoreceptor localization depends on specialized properties of these cytoskeletal elements.Citation26 How are photoreceptor molecules directed specifically to the D4 rootlet in wild-type cells? Mutations in the MLT1 (multi-eyed) gene allow photoreceptor localization and formation of supernumerary eyespots in the mother as well as daughter hemispheres of the cell. The mlt1 mutant exhibits enhanced sensitivity to the microtubule-destabilizing drug colchicine, lending support to the hypothesis that MLT1 affects the stability of the microtubule cytoskeleton.Citation27 Association of MLT1, a low-complexity protein with no identifiable domains, with either the mother or daughter basal body (or associated structures) may promote association of photoreceptor with the daughter rootlet and/or block localization to the mother hemisphere (), although other rootlet-associated factors or modifications likely play roles in establishing the specificity of the D4 rootlet. Trafficking of ChRs along the rootlet by plus-end directed motor proteins is one possible mechanism, although specific motor proteins responsible for photoreceptor transport have not yet been identified.
Examination of several Chlamydomonas reinhardtii mutants indicates that the D4 rootlet is also responsible for determining the equatorial placement of the eyespot along the anterior-posterior axis of the cell. The extent of acetylated microtubule length is diminished in mlt1 cells and corresponds with increased ChR1 accumulation near the basal bodies and more anteriorly-localized eyespots.Citation26 Conversely, longer microtubule rootlets in the cmu1 (cytoplasmic microtubules unorganized) mutant and the recently-identified pey1 (posterior eyespot) mutant correlated with posterior placement of the eyespot.Citation27 This cytoskeleton-mediated positioning process, however, may be more complex than it initially appears. Preliminary observations of dividing cells indicate that acetylation of the D4 rootlets extends to the extreme posterior of the cells before receding, while the nascent eyespots remain in an approximately equatorial position along the rootlet. Chlamydomonas reinhardtii harbors a homologue to the recentlyidentified microtubule acetyltransferase MEC-17,Citation28 (Joint Genome Institute C. reinhardtii version 4.0; www.chlamy.org), which may be an important factor in rootlet growth and regression during the transition to interphase. These data support the hypothesis that dynamic regulation of microtubule acetylation or other post-translational modifications by MLT1 and other factors during specific points in the cell cycle determines the final placement of the eyespot.
Eyespot Assembly Requires the Coordination of Multiple Cellular Compartments
What connects the positioning information of the D4 rootlet to the organization of the eyespot pigment granule stacks in the chloroplast? Analysis of eyespot mutants lacking chloroplast pigment granules revealed that the plasma membrane-localized photoreceptor ChR1 co-positions with a thioredoxin-family protein, EYE2, located in one of the membranes of the chloroplast envelope.Citation29 In the absence of pigment granules, both EYE2 and ChR1 were found together in patterns of spots or “stripes” along the D4 rootlet.Citation29 EYE2 localization to the specific area of chloroplast membrane in the eyespot may be guided by rootlet-associated cues in parallel with rootlet-microtubule-mediated photoreceptor localization; alternatively, formation of the EYE2 patch may be a downstream effect of photoreceptor aggregation at the site of eyespot assembly. Construction of a mutant in which expression of both eyespot channelrhodopsins (ChR1 and ChR2) are abolished would help distinguish between these possibilities. In either model, cytoplasmic elements (microtubule rootlet and associated proteins) act to define asymmetry in the chloroplast.
EYE2, in turn, is required for organization of the pigment granule arrays.Citation30,Citation31 The eye2 mutant retains asymmetricallylocalized ChR1 photoreceptor, but lacks the eyespot granule layers.Citation30 Analysis of another eyespot mutant, eye1, characterized by delayed pigment granule organizationCitation32,Citation33 demonstrated that the elliptical EYE2/ChR1 patch is established prior to organization of pigment granule arrays,Citation34 supportive of a model wherein EYE2 recruits pigment granules to the specialized area of chloroplast envelope, directing the site where the arrays assemble (). The localization patterns of ChR1 and EYE2 suggest that a stable link is established between these proteins during eyespot assembly, and that the pigment granules are required to maintain the elliptical shape of the EYE2 and photoreceptor patches. Proteins such as MIN1 are thought to participate in stabilization of the connections between membrane layers in the eyespot,Citation35 and likely contribute to maintaining the supramolecular organization of the compartments comprising the organelle.
Future Directions
The Chlamydomonas eyespot provides a fascinating arena for probing the interface of asymmetric cytoskeletal organization and organellar biology. Analysis of mutations in ChRs and microtubule-associated proteins should assist in elucidating the mechanism of photoreceptor localization, while further characterization of proteins such as MLT1 should augment our understanding of how asymmetry is propagated in Chlamydomonas as well as other eukaryotic systems. Future experiments characterizing and targeting regulators of rootlet microtubule stability should help address the mechanisms whereby the D4 rootlet assumes asymmetric properties. In addition, functional analysis of proteins identified in the eyespot proteomeCitation36 should shed light on the contributions of individual factors to establishment and maintenance of the numerous protein-protein and membrane interactions necessary for eyespot organization. Identification of regulators of both microtubule rootlet dynamics and eyespot genes will be essential for developing a comprehensive picture of the coordinated assembly and placement of this multicompartmental organelle.
Abbreviations
| D4 | = | daughter four-membered |
| ChR | = | channelrhodopsin |
Figures and Tables
Figure 1 Eyespot placement and assembly in Chlamydomonas. Left: Diagram showing cytoarchitecture of a Chlamydomonas cell. The basal bodies (small blue circles) nucleate the flagella and sets of two- and four-membered microtubule rootlets. The M2 and M4 rootlets are inherited from the mother cell, whereas the D2 and D4 rootlets are newly formed in the daughter cell. The asymmetrically-placed eyespot (large orange ellipse) is associated with the D4 rootlet and positioned near the cell equator. (A) Simplified working model of asymmetric photoreceptor localization. Channel-rhodopsin (ChR) photoreceptors traffic on endomembrane vesicles from the Golgi to the plasma membrane. MLT1 directs ChRs to the daughter side, where photoreceptors are transported by motor proteins (dark violet ellipses) along the D4 rootlet. (B) Simplified working model of eyespot assembly. ChRs form a patch in the plasma membrane. Rootlet or ChR-associated cues guide formation of a patch of EYE2 in either the inner or outer chloroplast envelope membrane, which nucleates formation of eyespot pigment granule arrays. Specialized proteins in the eyespot establish and maintain connections between the chloroplast envelope and plasma membrane. CE, chloroplast envelope; PM, plasma membrane.
References
- Dworkin J. Cellular polarity in prokaryotic organisms. Cold Spring Harb Perspect Biol 2009; 1:3368
- Vinogradova T, Miller PM, Kaverina I. Microtubule network asymmetry in motile cells: role of Golgi-derived array. Cell Cycle 2009; 8:2168 - 2174
- Snell WJ, Pan J, Wang Q. Cilia and flagella revealed: from flagellar assembly in Chlamydomonas to human obesity disorders. Cell 2004; 117:693 - 697
- Gönczy P. Mechanisms of asymmetric cell division: flies and worms pave the way. Nat Rev Mol Cell Biol 2008; 9:355 - 366
- Abrash EB, Bergmann DC. Asymmetric cell divisions: a view from plant development. Dev Cell 2009; 16:783 - 796
- Bornens M. Organelle positioning and cell polarity. Nat Rev Mol Cell Biol 2008; 9:874 - 886
- Li R, Gundersen GG. Beyond polymer polarity: how the cytoskeleton builds a polarized cell. Nat Rev Mol Cell Biol 2008; 9:860 - 873
- Harris EH. Chlamydomonas as a model organism. Annu Rev Plant Physiol Plant Mol Biol 2001; 52:363 - 406
- Merchant SS, Prochnik SE, Vallon O, Harris EH, Karpowicz SJ, Witman GB, et al. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 2007; 318:245 - 251
- LeDizet M, Piperno G. Cytoplasmic microtubules containing acetylated alpha-tubulin in Chlamydomonas reinhardtii—spatial arrangement and properties. J Cell Biol 1986; 103:13 - 22
- Foster KW, Smyth RD. Light antennas in phototactic algae. Microbiol Rev 1980; 44:572 - 630
- Kreimer G. The green algal eyespot apparatus: a primordial visual system and more?. Curr Genet 2009; 55:19 - 43
- Witman GB. Chlamydomonas phototaxis. Trends Cell Biol 1993; 3:403 - 408
- Melkonian M, Robenek H. Round FE, Chapman DJ. The eyespot apparatus of flagellated green algae: a critical review. Progress in Phycological Research 1984; 3:Bristol, UK Biopress Ltd. 193 - 268
- Nagel G, Ollig D, Fuhrmann M, Kateriya S, Musti AM, Bamberg E, et al. Channelrhodopsin-1: a light-gated proton channel in green algae. Science 2002; 296:2395 - 2398
- Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci USA 2003; 100:13940 - 13945
- Berthold P, Tsunoda SP, Ernst OP, Mages W, Gradmann D, Hegemann P. Channelrhodopsin-1 initiates phototaxis and photophobic responses in Chlamydomonas by immediate light-induced depolarization. Plant Cell 2008; 20:1665 - 1677
- Holmes JA, Dutcher SK. Cellular asymmetry in Chlamydomonas reinhardtii. J Cell Sci 1989; 94:273 - 285
- Ringo DL. Flagellar Motion and Fine Structure of Flagellar Apparatus in Chlamydomonas. J Cell Biol 1967; 33:543 - 571
- Moestrup Ø. On the phylogenetic validity of the flagellar apparatus in green algae and other chlorophyll A and B containing plants. Biosystems 1978; 10:117 - 144
- Dutcher SK. Elucidation of basal body and centriole functions in Chlamydomonas reinhardtii. Traffic 2003; 4:443 - 451
- Geimer S, Melkonian M. The ultrastructure of the Chlamydomonas reinhardtii basal apparatus: identification of an early marker of radial asymmetry inherent in the basal body. J Cell Sci 2004; 117:2663 - 2674
- Kamiya R, Witman GB. Submicromolar levels of calcium control the balance of beating between the two flagella in demembranated models of Chlamydomonas. J Cell Biol 1984; 98:97 - 107
- Ehler LL, Dutcher SK. Pharmacological and genetic evidence for a role of rootlet and phycoplast microtubules in the positioning and assembly of cleavage furrows in Chlamydomonas reinhardtii. Cell Motil Cytoskeleton 1998; 40:193 - 207
- Doonan JH, Grief C. Microtubule cycle in Chlamydomonas reinhardtii: an immunofluorescence study. Cell Motil Cytoskeleton 1987; 7:381 - 392
- Mittelmeier TM, Boyd JS, Lamb MR, Dieckmann CL. Asymmetric properties of the Chlamydomonas reinhardtii cytoskeleton direct rhodopsin photoreceptor localization. J Cell Biol 2011; 193:741 - 753
- Boyd JS, Gray MM, Thompson MD, Horst CJ, Dieckmann CL. The daughter four-membered microtubule rootlet determines anterior-posterior positioning of the eyespot in Chlamydomonas reinhardtii. Cytoskeleton (Hoboken) 2011; 68:459 - 469
- Akella JS, Wloga D, Kim J, Starostina NG, Lyons-Abbott S, Morrissette NS, et al. MEC-17 is an alpha-tubulin acetyltransferase. Nature 2010; 467:218 - 222
- Boyd JS, Mittelmeier TM, Lamb MR, Dieckmann CL. Thioredoxin-family protein EYE2 and ser/thr kinase EYE3 play interdependent roles in eyespot assembly. Mol Biol Cell 2011; 22:1421 - 1429
- Lamb MR, Dutcher SK, Worley CK, Dieckmann CL. Eyespot-assembly mutants in Chlamydomonas reinhardtii. Genetics 1999; 153:721 - 729
- Roberts DGW, Lamb MR, Dieckmann CL. Characterization of the EYE2 gene required for eyespot assembly in Chlamydomonas reinhardtii. Genetics 2001; 158:1037 - 1049
- Hartshorne JN. The function of the eyespot in Chlamydomonas. New Phytol 1953; 52:292 - 297
- Morel-Laurens NML, Feinleib ME. Photomovement in an “eyeless” mutant of Chlamydomonas. Photochem Photobiol 1983; 37:189 - 194
- Boyd JS. Eyespot assembly and positioning in Chlamydomonas reinhardtii 2011; Tucson, AZ University of Arizona 72 - 73 Ph.D. dissertation
- Mittelmeier TM, Berthold P, Danon A, Lamb MR, Levitan A, et al. C2 domain protein MIN1 promotes eyespot organization in Chlamydomonas reinhardtii. Euk Cell 2008; 7:2100 - 2112
- Schmidt M, Geßner G, Luff M, Heiland I, Wagner V, Kaminski M, et al. Proteomic analysis of the eyespot of Chlamydomonas reinhardtii provides novel insights into its components and tactic movements. Plant Cell 2006; 18:1908 - 1930