Abstract
Growth factors and their receptors are important for cellular migration as well as axonal guidance and myelination in the brain. They also play a key role in programmed cell death, and are implicated in a number of mental illnesses. Recently, we reported that healthy young adults who carry the T allele variant in the growth factor gene, NTRK1 (at location rs6336), had lower white matter integrity than non-carriers on diffusion images of the brain. Diffusion tensor imaging (DTI) revealed how this single nucleotide polymorphism affects white matter microstructure in human populations; DTI is also used to identify characteristic features of brain connectivity in typically developing children and in patients. Newly discovered links between neuroimaging measures and growth factors whose molecular neuroscience is well known offer an important step in understanding mechanisms that contribute to brain connectivity. Altered fiber connectivity may mediate the relationship between some genetic risk factors and a variety of mental illnesses.
Neurotrophins are a family of proteins that influence the migration, development and survival of neurons; in the central nervous system, these growth factors are mainly produced by neurons. They are taken up by other neurons that express the appropriate tropomyosin-related kinase (Trk) receptors. Three of the better-known neurotrophins are nerve growth factor (NGF), which binds with high affinity to the neurotrophic tyrosine kinase receptor 1 (NTRK1; also known as TrkA); brain derived neurotrophic factor (BDNF), which binds to NTRK2 (also known as TrkB), and neurotrophin 3 (NT3), which binds to both NTRK2 and NTRK3 (also known as TrkC). Our laboratory discovered that certain mental illness-associated genetic variants in BDNF, NTRK1, and NTRK3 are all related to diffusion tensor imaging (DTI) measures of white matter integrity in young healthy adults (aged 20–30).Citation1-Citation3 DTI scans are a special type of magnetic resonance image (MRI), sensitive to how water diffuses in the brain, rather than to the hydrogen content of tissues (which is the principle underlying standard anatomical MRI; ). The genetic variants we studied are single nucleotide polymorphisms – or SNPs – that are commonly carried, even in healthy human populations. SNPs are considered to be associated with mental illness if one of the alleles, or genetic forms, at that SNP is over-represented in patients based on large-scale genome-wide association studies. Several genetic variants in neurotrophins and their receptors are associated with mental illnesses including schizophrenia, bipolar disorder, and obsessive-compulsive disorder.Citation4-Citation7 These disorders frequently aggregate in families or co-occur within individuals.Citation8,Citation9 In addition, each of these disorders has previously been associated, at the group level, with identifiable differences in white matter microstructure (measured using DTI).Citation10-Citation12 When “risk genes” for mental illness are identified, their mechanism is not always clear, and it is critical to discover how the variants may combine to impact disease development. Knowledge of how a risk gene operates in the body, or the brain specifically, may improve personal risk assessment in high-risk individuals, encourage tailored treatment and prevention Citation13, and boost the power in disease studies through cohort stratification by relevant genetic differences.
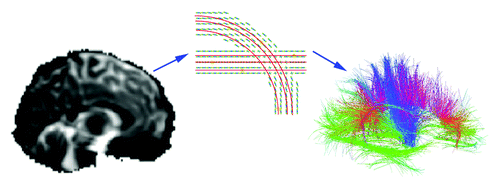
Figure 1. Diffusion tensor imaging and tract tracing. In whole brain tractography, diffusion weighted images (left panel) show the diffusion of water in specific directions (94 directions in the current study). A diffusion function (middle panel) can be reconstructed based on the sampling of diffusion in each direction, to identify axons and major tracts, as water tends to diffuse preferentially along tracts. Tract tracing algorithms can assemble the data into curves and bundles based on diffusion properties. In a three-dimensional map of the recovered fibers (right panel), a color code indicates the fiber directions. These fibers may be grouped into bundles and their integrity and connectivity can be evaluated. This image is adapted from ref. Citation29.
We recently discovered a relationship between white matter integrity and an NTRK1 genetic variant (rs6336), previously associated with schizophrenia.Citation6,Citation14 This link is promising as it connects measures of fiber integrity on a brain scan to decades of molecular and neurodevelopmental work on growth factors—specifically, nerve growth factor (NGF) and its receptor. The NTRK1 gene encodes a high affinity receptor for NGF, but approximately 8% of Caucasians from Northern and Western European ancestryCitation15 carry a different allele T (at locus rs6336 in the NTRK1 gene), which also may be associated with schizophrenia risk. Intriguingly, the reported T allele frequency was zero in Han Chinese in Beijing, Japanese in Tokyo, and in Yoruban in Ibadan, Nigeria.Citation15 By scanning the brain with diffusion MRI, we were able to identify one specific way in which this allele disrupts the living human brain—one step toward a more mechanistic understanding of this gene variant.
We found that the T allele of rs6336 (NTRK1-T) was associated with decreased fractional anisotropy (FA) and increased radial diffusivity (Drad) as determined using DTI.Citation3 FA is a widely used and widely accepted measure of white matter integrity. It measures the extent to which water diffusion is directionally constrained. Higher values usually, but not always, indicate greater brain fiber myelination.Citation16 More constrained diffusion indicates greater myelination as the myelin sheaths act as barriers to water diffusion, making the water molecules diffuse preferentially along axons rather than across them. Drad represents diffusion perpendicular to the axon fiber. Higher Drad may indicate less dense axonal packing, a lesser degree of myelination, a greater mean axon diameter, higher permeability of axonal fibers, or a combination of these.Citation17 There are at least two possible explanations for why the carriers of this genetic variant on average have less directional diffusion in their white matter.
The lower FA and higher Drad we found in T-allele carriers may relate to programmed cell death during development. Early in brain development, neurotrophins are transported in a retrograde fashion along the axon to the soma of neurons. Only those neurons that bind and transport the neurotrophins survive programmed cell death (as reviewed in ref. Citation18). Neurons that express the NTRK1 or NTRK3 receptors in both the central and peripheral nervous system die within days in vitro unless exposed to their ligands, NGF and NT3, respectively.Citation19 Because of this, genetic polymorphisms that increase the ratio of NTRK1 or NTRK3 receptors to available NGF or NT3 may increase neuronal death developmentally, resulting in a decrease in the density of fibers connecting specific brain regions. NTRK1-T is a missense mutation—in other words the resulting codon in the genome codes for a different amino acid. The minor allele, or “risk allele,” T codes for tyrosine, rather than the histidine encoded by the more common C allele at position 598 within the kinase domain.Citation6 It is unclear whether the mutation increases or decreases expression of the NTRK1 receptor in the brain. It is possible though that the decreased fractional anisotropy and increased radial diffusivity we found in NTRK1-T carriers previouslyCitation3 may reflect such a decrease, as lower fiber packing allows for more diffusion perpendicular to the length of an axon.
Since our previous NTRK1 report was published,Citation3 we have been studying how the same variant affects patterns of brain connectivity, so here we are taking the opportunity to report an update. In a subset of 359 of the 391 subjects (330 C/C, 27 C/T; 2 T/T) from our prior study,Citation3 we examined cortical connectivity by NTRK1 genotype across the brain’s white matter using the analysis methods detailed previously.Citation20 Participant information is available in our earlier publication Citation3. Cortical connectivity was defined as the estimated proportion of fibers in the brain that connect one cortical region to another—compared with total number of detected fibers in a given subject. Briefly, cortical regions were delineated on each subject’s anatomical brain scans (T1-weighted) in a common template space. Using a method called whole-brain tractography, we were able to build a map of fiber trajectories in the brain by following the paths of maximum water diffusion. The connections between pairs of cortical and subcortical regions were identified and counted. To ensure that only consistently present connections were evaluated, the only connections compared by genotype were those found in at least 95% of the study participants.
Connectivity between each pair of cortical regions was compared by genotype group. In this kind of analysis, it is possible to define a useful measure of anatomical connectivity in terms of the density of recovered fibers connecting any pair of brain regions, which is what we did here.
There were 32 subjects excluded from the current analysis but included in our previous study of NTRK1-T differences in DTI FA.Citation3 For 21 of them, the cortical segmentation was inadequate, for 9, the whole-brain tractography was too sparse and failed to adequately cover the full brain, and for 2, other technical difficulties made the connectivity data unusable.
In this new analysis, NTRK1-T allele carriers had a higher relative density of fibers in the anatomical pathway between the left superior parietal cortex and supramarginal gyrus. This was not a region with FA differences between genotypes.Citation3 However, our connectivity analysis lacked significant support for lower relative fiber density in our NTRK1-T carriers. This suggests that either differences in fiber density are not the main driving component of our FA differences, or that genotype differences were associated with fairly uniform changes in fiber density across the brain, in which case our relative connectivity analysis would not detect differences. NTRK2, the high affinity receptor for BDNF, is not implicated in cell death,Citation19 so differences in programmed cell death processes are unlikely to explain the relationship our laboratory found between the Val allele at BDNF rs6265 and lower DTI FA.Citation1 Cortical thickness and volume may not be directly correlated with neuronal count,Citation21, Citation22 so neuronal loss related to programmed cell death may or may not be reflected in measures of cortical thickness or volume.
NTRK1-T may cause the FA differences we saw by influencing axon myelination. Neurotrophins and their receptors have a well-established role in peripheral nervous system myelination.Citation23 Additionally, oligodendrocytes, a major component of myelin in the central nervous system express and secrete neurotrophins. The presence of NGF in dorsal root ganglion (DRG)-oligodendrocyte progenitor cell (OPC) co-culture decreases myelination. Its presence reduces the expression of the oligodendrocyte marker: 2',3'-cyclic nucleotide 3'-phosphodiesterase (CNPase), and markers of oligodendrocyte maturation: myelin-associated glycoprotein (MAG) and myelin basic protein (MBP). NGF also prevents a large proportion of oligodendrocytes from extending processes and myelinating axons. These prior findings indicate that NGF reduces oligodendrocyte generation and maturation and also impairs myelination by those oligodendrocytes, once the oligodendrocytes are mature.Citation24 The effect is mediated by activating NTRK1 receptors in neuronal (rather than glial) cells. This suggests that NGF inhibits myelination by regulating neuronal signaling rather than acting directly on the glia.Citation24 NGF and NTRK1 exert their effect on myelination in part by helping to regulate LRR (leucine-rich repeat) and Ig domain-containing Nogo receptor-interacting protein (LINGO-1), which is a strong axonal inhibitor of oligodendrocyte differentiation and myelination.Citation25 If the NTRK1-T variant were therefore to increase expression of the neuronal NTRK1 receptors in the presence of excess ligand (such as NGF), it could reduce brain myelination, which could explain our lower FA in NTRK1-T carriers. To elucidate the mechanisms behind our findings, more information is needed on how the variant relates to NTRK1 receptor expression in the brain.
Finally, NTRK1-T could increase axonal diameter (caliber). When stimulated by neurotrophins, Trk kinases such as NTRK1 activate intracellular pathways. One such pathway includes phosphatidylinositol-3′-kinase (PI3K) and the protein kinase Akt, both of which have been shown to increase axonal diameter in culture.Citation26 Such an increase in diameter could explain the greater Drad we found in NTRK1-T carriers if the mutation resulted in greater NTRK1 signaling. However, increased axonal diameter would likely also be associated with increased Dax (ref. Citation27) (diffusion parallel to the axonal fiber), which we did not find in our previous study.Citation3 Therefore, an increase in axonal diameter related to increased NTRK1 signaling is a less likely explanation for our FA results.
In summary, we previously found that a missense mutation in NTRK1 at rs6336 related to lower brain white matter integrity in carriers of the T allele. Carriers of this allele may, on average, exhibit differences in programmed neuronal death during development or differences in central nervous system myelination, both of which are influenced by activation of NTRK1 receptors by NGF. The next step in this work will be to compare the mean number of regional axonal fibers in a larger sample of NTRK1-T carriers to non-carriers, and to determine how the variant affects NTRK1 expression in central nervous system neurons. Once we understand how variants in growth factor genes affect a neuronal pathway, we may also evaluate the composite effect on white matter integrity of numerous polymorphisms in the same pathway using a tool designed to evaluate multiple variants together while correcting for the effects of each variant on all the others.Citation28 Such information may help predict individual risk for brain disease, and may inform treatment efforts by stratifying those receiving treatment into genetic strata with different white matter architecture.
| Abbreviations: | ||
| FA | = | fractional anisotropy |
| DTI | = | diffusion tensor imaging |
| Drad | = | radial diffusivity |
| Dax | = | axial diffusivity |
| NTRK1 | = | neurotrophic tyrosine kinase receptor 1 |
| TrkA | = | tropomyosin-related kinase receptor A |
| BDNF | = | brain derived neurotrophic factor |
| NT3 | = | neurotrophin 3 |
| NTRK1-T | = | allele T at NTRK1 variant rs6336 |
| ODFs | = | orientation distribution functions |
| DRG | = | dorsal root ganglion |
| OPC | = | oligodendrocyte progenitor cell |
| CNPase | = | 2’,3′-cyclic nucleotide 3′-phosphodiesterase |
| MAG | = | myelin-associated glycoprotein |
| MBP | = | myelin basic protein |
| LINGO-1 | = | LRR (leucine-rich repeat) and Ig domain-containing Nogo receptor-interacting protein |
| PI3K | = | phosphatidylinositol-3′-kinase |
| Akt | = | protein kinase B |
Acknowledgments
We thank the twins and siblings for their participation. In Brisbane, we thank Marlene Grace and Ann Eldridge for twin recruitment, Aiman Al Najjar and other radiographers for scanning, Kerrie McAloney and Daniel Park for research support, and staff in the Molecular Epidemiology Laboratory for DNA sample processing and preparation. This study was supported by the National Institute of Child Health and Human Development (R01 HD050735), and the National Health and Medical Research Council (NHMRC 486682), Australia. Genotyping was supported by the NHMRC (grant 389875). Additional support was provided by NIH R01 grants AG040060, EB008432, EB008281, and EB007813. MNB was funded in part by the NIH (P50 AG-16570) and by the UCLA Easton Consortium for Biomarker and Drug Discovery in Alzheimer Disease. G.Z. is supported by an ARC Future Fellowship (FT0991634). We thank Emily Dennis for preparing the figure included here.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Chiang MC, Barysheva M, Toga AW, Medland SE, Hansell NK, James MR, et al. BDNF gene effects on brain circuitry replicated in 455 twins. Neuroimage 2011; 55:448 - 54; http://dx.doi.org/10.1016/j.neuroimage.2010.12.053; PMID: 21195196
- Braskie MN, Kohannim O, Jahanshad N, Chiang MC, Barysheva M, Johnson K, et al. Genetic variation within NTRK3 influences white matter integrity in healthy young adults. Imaging Genetics. Irvine, CA, USA, 2012.
- Braskie MN, Jahanshad N, Stein JL, Barysheva M, Johnson K, McMahon KL, et al. Relationship of a variant in the NTRK1 gene to white matter microstructure in young adults. J Neurosci 2012; 32:5964 - 72; http://dx.doi.org/10.1523/JNEUROSCI.5561-11.2012; PMID: 22539856
- Alonso P, Gratacòs M, Menchón JM, Segalàs C, González JR, Labad J, et al. Genetic susceptibility to obsessive-compulsive hoarding: the contribution of neurotrophic tyrosine kinase receptor type 3 gene. Genes Brain Behav 2008; 7:778 - 85; http://dx.doi.org/10.1111/j.1601-183X.2008.00418.x; PMID: 18616610
- Athanasiu L, Mattingsdal M, Melle I, Inderhaug E, Lien T, Agartz I, et al. Intron 12 in NTRK3 is associated with bipolar disorder. Psychiatry Res 2011; 185:358 - 62; http://dx.doi.org/10.1016/j.psychres.2010.05.011; PMID: 20554328
- van Schijndel JE, van Loo KM, van Zweeden M, Djurovic S, Andreassen OA, Hansen T, et al. Three-cohort targeted gene screening reveals a non-synonymous TRKA polymorphism associated with schizophrenia. J Psychiatr Res 2009; 43:1195 - 9; http://dx.doi.org/10.1016/j.jpsychires.2009.04.006; PMID: 19435634
- Pae CU, Chiesa A, Porcelli S, Han C, Patkar AA, Lee SJ, et al. Influence of BDNF variants on diagnosis and response to treatment in patients with major depression, bipolar disorder and schizophrenia. Neuropsychobiology 2012; 65:1 - 11; http://dx.doi.org/10.1159/000327605; PMID: 22094229
- Joshi G, Wozniak J, Petty C, Vivas F, Yorks D, Biederman J, et al. Clinical characteristics of comorbid obsessive-compulsive disorder and bipolar disorder in children and adolescents. Bipolar Disord 2010; 12:185 - 95; http://dx.doi.org/10.1111/j.1399-5618.2010.00795.x; PMID: 20402711
- Bramon E, Sham PC. The common genetic liability between schizophrenia and bipolar disorder: a review. Curr Psychiatry Rep 2001; 3:332 - 7; http://dx.doi.org/10.1007/s11920-001-0030-1; PMID: 11470041
- Vederine FE, Wessa M, Leboyer M, Houenou J. A meta-analysis of whole-brain diffusion tensor imaging studies in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35:1820 - 6; http://dx.doi.org/10.1016/j.pnpbp.2011.05.009; PMID: 21624424
- Ellison-Wright I, Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res 2009; 108:3 - 10; http://dx.doi.org/10.1016/j.schres.2008.11.021; PMID: 19128945
- White T, Nelson M, Lim KO. Diffusion tensor imaging in psychiatric disorders. Top Magn Reson Imaging 2008; 19:97 - 109; http://dx.doi.org/10.1097/RMR.0b013e3181809f1e; PMID: 19363432
- Mrazek DA. Psychiatric Pharmacogenomics. New York: Oxford University Press, 2010.
- Van Schijndel JE, Van Zweeden M, Van Loo KM, Djurovic S, Andreassen OA, Hansen T, et al. Dual association of a TRKA polymorphism with schizophrenia. Psychiatr Genet 2011; 21:125 - 31; http://dx.doi.org/10.1097/YPG.0b013e3283437194; PMID: 21317683
- Thorisson GA, Smith AV, Krishnan L, Stein LD. The International HapMap Project Web site. Genome Res 2005; 15:1592 - 3; http://dx.doi.org/10.1101/gr.4413105; PMID: 16251469
- Thomason ME, Thompson PM. Diffusion imaging, white matter, and psychopathology. Annu Rev Clin Psychol 2011; 7:63 - 85; http://dx.doi.org/10.1146/annurev-clinpsy-032210-104507; PMID: 21219189
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed 2002; 15:435 - 55; http://dx.doi.org/10.1002/nbm.782; PMID: 12489094
- Skaper SD. The neurotrophin family of neurotrophic factors: an overview. Methods Mol Biol 2012; 846:1 - 12; http://dx.doi.org/10.1007/978-1-61779-536-7_1; PMID: 22367796
- Nikoletopoulou V, Lickert H, Frade JM, Rencurel C, Giallonardo P, Zhang L, et al. Neurotrophin receptors TrkA and TrkC cause neuronal death whereas TrkB does not. Nature 2010; 467:59 - 63; http://dx.doi.org/10.1038/nature09336; PMID: 20811452
- Jahanshad N, Hibar DP, Ryles A, Toga AW, McMahon KL, de Zubicaray GI, et al. Discovery of Genes That Affect Human Brain Connectivity: A Genome-Wide Analysis of the Connectome. Proc IEEE Int Symp Biomed Imaging 2012:542-5.
- Bothwell S, Meredith GE, Phillips J, Staunton H, Doherty C, Grigorenko E, et al. Neuronal hypertrophy in the neocortex of patients with temporal lobe epilepsy. J Neurosci 2001; 21:4789 - 800; PMID: 11425906
- Freeman SH, Kandel R, Cruz L, Rozkalne A, Newell K, Frosch MP, et al. Preservation of neuronal number despite age-related cortical brain atrophy in elderly subjects without Alzheimer disease. J Neuropathol Exp Neurol 2008; 67:1205 - 12; http://dx.doi.org/10.1097/NEN.0b013e31818fc72f; PMID: 19018241
- Xiao J, Kilpatrick TJ, Murray SS. The role of neurotrophins in the regulation of myelin development. Neurosignals 2009; 17:265 - 76; http://dx.doi.org/10.1159/000231893; PMID: 19816063
- Chan JR, Watkins TA, Cosgaya JM, Zhang C, Chen L, Reichardt LF, et al. NGF controls axonal receptivity to myelination by Schwann cells or oligodendrocytes. Neuron 2004; 43:183 - 91; http://dx.doi.org/10.1016/j.neuron.2004.06.024; PMID: 15260955
- Lee X, Yang Z, Shao Z, Rosenberg SS, Levesque M, Pepinsky RB, et al. NGF regulates the expression of axonal LINGO-1 to inhibit oligodendrocyte differentiation and myelination. J Neurosci 2007; 27:220 - 5; http://dx.doi.org/10.1523/JNEUROSCI.4175-06.2007; PMID: 17202489
- Markus A, Zhong J, Snider WD. Raf and akt mediate distinct aspects of sensory axon growth. Neuron 2002; 35:65 - 76; http://dx.doi.org/10.1016/S0896-6273(02)00752-3; PMID: 12123609
- Harsan LA, Poulet P, Guignard B, Steibel J, Parizel N, de Sousa PL, et al. Brain dysmyelination and recovery assessment by noninvasive in vivo diffusion tensor magnetic resonance imaging. J Neurosci Res 2006; 83:392 - 402; http://dx.doi.org/10.1002/jnr.20742; PMID: 16397901
- Kohannim O, Jahanshad N, Braskie MN, Stein JL, Chiang MC, Reese AH, et al. Predicting white matter integrity from multiple common genetic variants. Neuropsychopharmacology 2012; 37:2012 - 9; http://dx.doi.org/10.1038/npp.2012.49; PMID: 22510721
- Aganj I, Lenglet C, Jahanshad N, Yacoub E, Harel N, Thompson PM, et al. A Hough transform global probabilistic approach to multiple-subject diffusion MRI tractography. Med Image Anal 2011; 15:414 - 25; http://dx.doi.org/10.1016/j.media.2011.01.003; PMID: 21376655