Abstract
Curiosity has driven humankind to explore and conquer space. However, today, space research is not a means to relieve this curiosity anymore, but instead has turned into a need. To support the crew in distant expeditions, supplies should either be delivered from the Earth, or prepared for short durations through physiochemical methods aboard the space station. Thus, research continues to devise reliable regenerative systems. Biological life support systems may be the only answer to human autonomy in outposts beyond Earth. For construction of an artificial extraterrestrial ecosystem, it is necessary to search for highly adaptable super-organisms capable of growth in harsh space environments. Indeed, a number of organisms have been proposed for cultivation in space. Meanwhile, some manipulations can be done to increase their photosynthetic potential and stress tolerance. Genetic manipulation and screening of plants, microalgae and cyanobacteria is currently a fascinating topic in space bioengineering. In this commentary, we will provide a viewpoint on the realities, limitations and promises in designing biological life support system based on engineered and/or selected green organism. Special focus will be devoted to the engineering of key photosynthetic enzymes in pioneer green organisms and their potential use in establishment of transgenic photobioreactors in space.
Introduction
Foundation of a multipurpose and reliable life support system (LSS) is a necessity in providing astronauts with essential requirements in long-term space missions. This is in line with NASA Strategic Plan to extend and sustain human activities across the solar system and to create the innovative new technologies for exploration, science and economic future. Based on a recent report from NASA Marshall Space Flight Center, each average individual human being will need 0.84 kg oxygen, 0.62 kg food solids and a total of 29.00 kg water every day and in return will produce a total of 30.60 kg waste (including carbon dioxide, respiration and perspiration water, food preparation, latent water, urine, urine flush water, feces water, sweat solids, urine solids, feces solids, hygiene water and clothes water wash).Citation1 Autonomous biological LSS (BLSS) is believed to be the key to human long-term presence in space. Such a system will require a multispecies combination of higher plants, microorganisms, and physiochemical processes for revitalization of air and water, waste processing and definition of appropriate food webs. Designing probiotics with immunological benefitsCitation2 has also been proposed for replenishing the microbiome of astronauts,Citation3 which may open a new chapter in designing future BLSS.
Green organisms are solar-powered and self-perpetuating, a fact which reduces the need for expensive re-supply missions. Plants are capable of supplying humans with food and oxygen and mitigating carbon dioxide. Nonetheless, not all plants can withstand the harsh conditions of space such as varying temperatures, drought, radiations, exposure to heavy metals, microgravity and high carbon dioxide concentrations. Although many projects have been launched such as Herb Ward, BIOS project, Biosphere 2, Micro-Ecological Life Support System Alternative (MELISSA), Closed Ecology Experiment Facilities, Controlled Ecological Life Support Systems, BIO-plex (never completed), Salad Machine and Advanced LSS (ALSS), majority of these projects have been conducted in small scale and in contained environments. Establishing large hydroponic or aeroponic greenhouses is difficult due to inadequacy of light, microgravity, irrigation and accommodation issues. Since most of these plants cannot tolerate real space exposure, search continues to find super-species from extreme environments around the globe. There are terrestrial examples of successful adaptation on Earth such as species tolerant to low atmospheric pressure (Alpine), low temperature (Arctic species, Araceae), drought (Tortula ruralis, Physcomitrella), and those tolerant to high radiation such as Deinococcus radiodurans.Citation4 It is surmised that making an Earth-like analog in space will end up in a fiasco, unless lessons are learned from the evolution of life on Earth.
Classical breeding, involving crossbreeding and screening of populations, is slow and hampered by labor-intensiveness. Thus, genetic manipulation toolkits hold promise in making sophisticated green organisms resistant to extreme conditions in a more realistic and foreseeable time frame. In this commentary, three strategies will be presented to enrich the genetic reservoir of plants and microalgae to increase their resistance and photosynthetic yields, (1) screening of resistant organism from extreme environments on the Earth, (2) adaptation of organisms to harsh condition and (3) genetic manipulation.
Our knowledge about the origin and evolution of photosynthesis can advance our understanding of the origin of life. Life on Earth probably dates back to 3.8 billion years ago. However, scientists have hypothesized that life may have existed earlier on other planets and was translated to Earth by a meteorite.Citation5 Either way, today we know that the atmosphere has been produced by a phenomenon known as oxygenic photosynthesis, before which the Earth was anoxic. A great deal of atmospheric oxygen is produced not by plants, but by the photosynthetic cyanobacteria and microalgae in oceans, seas and lakes (). Establishment of an Earth-like atmosphere in short time will be feasible through construction of closed environments in space. The strategy to accelerate this process is screening candidate organisms and manipulating them to create super-species with higher photosynthetic efficiency and higher adaptability to grow in extraterrestrial environments. Apart from improving the photosynthetic efficiency, genetic manipulation of crops that are better suited to a spaceflight environment (e.g., dwarfed crops, or those that have high harvest indexes and grow well under artificial light, reduced atmospheric pressures or elevated carbon dioxide concentrations)Citation6 remain promising research topics to explore.
Figure 1. Salt Lake Urmia, one of the UNESCO Biosphere Reserves in Iran contains more than 300 g per liter salt and can thus be considered a region with astrobiological significance. The lake is home to several ancient photosynthetic organisms (Photo taken by authors in August 2012; Geographical position: 37.769642, 45.318928).

In this line, studying the evolution machinery in the Earth will help us reverse-genetic engineer green organisms to construct an artificial atmosphere as well as an autonomous food web in space. For instance, robust photosynthetic enzymes of ancient extremophile photosynthetic microorganism can be employed to enrich the genetic reservoir in candidate plants and microalgae.
Candidate Green Organisms for Integration in BLSS
Plants
Plants are generally regarded as essential elements of any successful BLSS. In recent centuries, advances in plant genetic breeding have brought about significant progress in increasing crop yields. Although many investigations have focused on designing hardwares to sustain plant growth in space, little work has been done to genetically engineer plants for exploitation in BLSS. Thousands of cultivars of each major crop plants (each adapted to a unique habitat on our planet) as well as national germplasm collections provide us with the genetic diversity to explore and engineer.Citation7 Lettuce, kale, turnip, Swiss chard, endive, dandelion, cabbage, cauliflower, radish, New Zealand spinach, tampala and sweet potato were the first crops chosen for use in BLSS.Citation8 According to Wright Patterson Air Force Base symposium, selection criteria included the ability of growth under low light intensity, compact size, high productivity, and osmotolerance.Citation9 Other factors such as compatibility with other components of BLSS and humans, reasonable duration of growth cycle, palatability, nutritional value and adaptability to the space condition need to be taken into consideration as well.
Although a large number of studies have been conducted to investigate the effect of microgravity on plants, few plants have been grown in space for revitalization of air and production of biomass to sustain human life. In 1970, Spyrodela polyrhiza and later potato and wheat, were used with the purpose of photosynthetic gas exchange.Citation10,Citation11 Other successful studies reported the production of wheat and potato biomass in space.Citation12-Citation14 Elevated ethylene levels hindered the development of wheat seeds in Russian Mir Space Station.Citation12,Citation14 In the PESTO experiment on successive plantings of wheat on the International Space Station, elegant gas exchange was noted.Citation15
The plants have adapted with respect to different environmental conditions on the Earth.Citation16 I; C3 plants have low photosynthetic efficiency due to photorespiration. However, they are resistant to extremely cold situations.Citation17 Wheat is among C3 plants and has been widely considered as an attractive candidate for cultivation in space. II; On the other hand, owing to the higher density of mesophyll cells, C4 plants such as maize have a higher photosynthetic efficiency, since in such a compact environment where little oxygen diffusion happens, Rubisco enzyme will solely act as a carboxylase and its oxygenase function will be lost.Citation18 The origin of C4 plants goes back to around 30 million years ago. After migration of C4 metabolism from shady forests to open lands, high sunlight radiation gave C4 plants an advantage over C3.Citation19 Increased resistance to water stress allowed C4 photosynthesis to more easily reside arid lands. III; Crassulacean Acid Metabolism (CAM) photosynthesis is a special carbon fixation pathway, evolved in response to adaptation of a number of plants to arid conditions. CAM plants have adapted to photosynthesis in dry environments.Citation20 These plants absorb carbon dioxide in dark and their photosynthetic function relies solely on the carbon dioxide stored at night. Water loss is minimized by closing stomata during the day. While C3 plants lose 97% of their water uptake by transpiration, plants employing CAM (e.g., coconut) are able to withstand extremely dry situations.Citation21 Although such plants can be employed in dry extraterrestrial environments, they have low level of photosynthesis. The photosynthetic properties and resistance profile of C3, C4 and CAM plants to various environmental conditions have been presented in .
Table 1. Comparison of C3, C4, and CAM plants in photosynthetic efficiency and resistance profile
Thus, none of these plants can be ideal for cultivation in dry environments with highly fluctuating temperatures. However, having been inspired by all these photosynthetic types, and using genetic engineering tools, we can sum up the advantages from all these organisms into making super-species suitable for cultivation in BLSS. The increase in understanding C4 photosynthesis has enabled researchers to engineer primary carbon metabolism in C3 plants by expression of C4 enzymes at high levels in specific locations in leaves of C3 plants. For example, the NADP-malic enzyme type C4 dicot Flaveria bidentis (L.) Kuntze has been transformed with several gene constructs to decrease the expression of certain enzymesCitation22 including Rubisco, pyruvate, Pi dikinase and NADP malate dehydrogenase. The majority of these manipulations can be performed on the key photosynthetic enzymes.
Genetic manipulation of key photosynthetic enzymes in plants
Rubisco
Rubisco or ribulose bisphosphate carboxylase/oxygenase, the most abundant enzyme on our planet helps capture carbon from atmosphere and thus plays a fundamental role in photosynthesis. The extensively investigated Rubisco is currently considered as one of the leading targets for genetic engineering to improve the photosynthetic efficiency of green organisms.Citation23 Both carboxylation and oxygenation reactions are catalyzed at the same active site on Rubisco which makes carbon dioxide and oxygen competitive substrates.Citation24 There is general consensus that reducing the inhibitory effect of oxygen can enhance the photosynthetic efficiency and, subsequently, the productivity of plants. It has also been hypothesized that carbon dioxide enrichment can increase crop yield by enhancing photosynthetic efficiency even at a global scale.Citation25 Although in the absence of oxygen in space, Rubisco will function only as a carboxylase, through construction of a closed ecosystem or air cabin, oxygen diffusion to the space between mesophyll cells will definitely affect the activity of Rubisco. Furthermore, microgravity will reduce the density of tissues and will increase the intracellular space, allowing more oxygen to come in contact with the Rubisco and influence its function. Thus, studying Rubisco evolution can provide us with the knowledge to manipulate Rubisco in higher plants to produce genetically modified plants harboring Rubisco enzymes with more carboxylase function than oxygense.
Research can also focus on elucidating other green organisms embedded with better Rubisco machineries. For example, the red alga Galdieria partita has one of the highest reported values for Rubisco specificity factor, almost 3-times greater than that of most crop plants.Citation26 There have been several attempts to select green organisms with higher specificity factors through maintaining them at above the compensation point, in which the species with relatively low rates of photorespiration thrive while those with relatively high rates of photorespiration die. Research has also been directed to increase the amount of this enzyme in green organisms by overexpressing the Rubisco gene. Details of these methods and associated references have been reviewed by Parry et al.Citation27
In the early Earth, the specificity of Rubisco for carbon dioxide may have been higher, since the early Earth largely contained carbon dioxide and only little oxygen. Rubsico can thus be isolated from archaic microorganisms and introduced into green candidates. Furthermore, engineering Rubisco activase, or increasing its stability holds promise for enhancing the adaptability of plants in stressful or extreme environments.Citation27 However, researchers must have an eye on the hypothesis that “genetic engineering to improve Rubisco might lead to a productivity runaway that removes all atmospheric carbon dioxide.”Citation5
Carbonic anhydrase
Carbonic anhydrase is another key photosynthetic metalloenzyme which catalyzes the rapid interconversion of carbon dioxide to bicarbonate and is responsible for delivery of atmospheric carbon dioxide to Rubisco. In microgravity in which the cellular density in plant leaves is lower than that of Earth, the presence of a robust carbonic anhydrase can capture high amounts of carbon dioxide and enhance photosynthetic function. This enzyme is of utmost importance in CAM plants. However, naturally, carbonic anhydrase has low activity in C3 and C4 plants.Citation28 The amount and robustness of carbonic anhydrase is higher in microalgae. Thus, these enzymes can be engrafted into plants to enhance their photosynthetic efficiency.
Pros and cons of plant in BLSS
From a more realistic point of view, there is little possibility that plants can establish a successful BLSS alone. A number of major drawbacks exist: First, it may not be practical to take large number of plants to off-planet stations and if so, a large surface area will be needed to accommodate them. For example, based on the best photosynthetic rates from studies on gas exchange of potato stands for bioregenerative life support, about 25 m2 of potato plants under continuous cultivation are required to remove the carbon dioxide and supply oxygen for one person.Citation29 The oxygen need of one human can be provided by 11 m2 of wheat grown at high light intensity.Citation30 This is while 17 L of the microalga Chlorella culture spread in a thin layer with light-receiving surface area of 8 m2 is sufficient for gas exchange of an average human subject.Citation31 Besides, super-elevation of the carbon dioxide concentration to 5000–10000 ppm which can occur in spacecraft, sometimes has negative and/or toxic effects on plant growth or seed yield in some species.Citation32 Trimming, watering and taking care of these plants can turn into a daily chore for astronauts. Other problems such as lack of adequate light and difficulty of supplying water to plants in microgravity also exist. Besides, since the nutritional composition of a single crop is not adequate for a healthy diet, different species may need to be incorporated. Another inconsistency is that almost half of the plant biomass is inedible. There are also concerns about the damages that may be induced by freezing and UV-B radiation in space. Sewage water recycling is a serious problem of any LSS and though plants have been proposed as means to recycle water, plants used in hydroponics are sensitive to pH changes and inorganic toxic elements. Taken altogether, it seems that other green organisms can be used along with higher plants to increase the functionality of BLSS ().
Microalgae as alternative green organisms
Microfossil records indicate that cyanobacteria and microalgae were the earliest residents of the Earth. Some of these organisms have the capability to grow in extreme environments on our planet () and even in simulated conditions of Mars and Moon.Citation33 They have been shown to survive in habitats with a wide range of salinity, pH, heavy metal, light intensity, UV radiation and extreme temperatures. Million years ago, photosynthetic microorganisms started to generate the atmospheric oxygenCitation34 and can thus be integrated into BLSS as functional components in revitalization of air and supplying food.
Microalgae are microscopic unicellular photoautotrophic organisms () with various industrial, pharmaceutical and nutritional applications and have recently come into attention in molecular farming.Citation35,Citation36 Microalgae have also been incorporated in several BLSSs because of high growth rate and little growth requirements, simple cultivation and relatively easy collection. Among other microalgae, Spirulina platensis is the most commonly used organism in BLSS owing to its rich nutritional merit.Citation37 Not only can microalgae function in nutrition and air revitalization but also in waste water treatment and producing biofuel. So far, in BLSS studies, microalgae have only been considered for revitalization of air and providing food, and the capability of microalgae in production of biofuels including biohydrogen has been ignored. This functionality can be regarded as an added benefit.
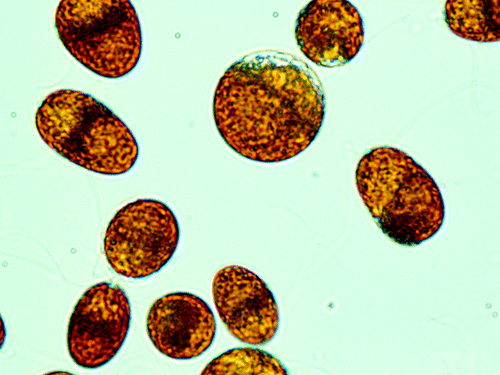
Figure 2. The microalgae Dunaliella urmiana harboring several photosynthetic pigments has adapted to the stressful life in various habitats including Salt Lake Urmia (Isolated and characterized by authors; × 100 magnification under Olympus Invert Microscope).
Protein and biomass quality and biological value of various microalgae including Chlorella, Chlamydomonas, Spirulina and Euglena has been calculated in a study by Antonian et al.Citation38 In this study, the high biological value of the algal biomass was demonstrated in animal experiments and the lack of its toxic or other adverse effects was confirmed. The authors concluded that the quantity and quality of proteins from the unicellular microalgae indicate their high nutritional value and applicability to BLSS.
Microalgae possess a set of vital enzymes which can be used in higher plants to increase the photosynthetic yield as well as resistance to space environment. Microalgal carbonic anhydrase is more robust than that of plants and can thus be cloned into other green organisms. Other important genes involved in carotenoid synthesis such as lycopene cyclase (lyc) and phytoene desaturase (pds) can be transferred to higher plants to confer resistance against high solar radiation and to protect chlorophyle molecules. On the other hand, these genes can be manipulated to increase the carotenoid content of the microalgal biomass which upon consumption can partly protect astronauts against radiations.
Ability of microalgae to adapt to harsh environments
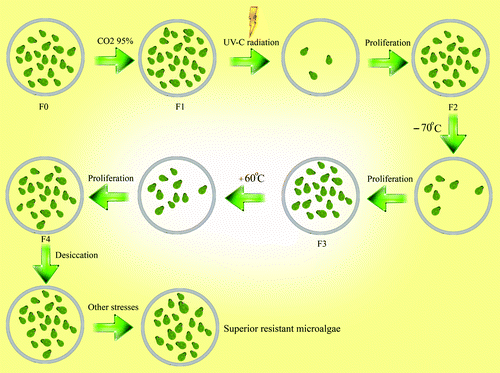
Crossing over and mutations are random phenomena during replication which contribute to adaptation of an organism to changing conditions, unusual and/or extreme environments by acquiring new genotypic and/or phenotypic traits. Unlike plants, microorganisms (including microalgae) are hypermutable and have high crossing over and mutation rate in response to new stressors. The higher rate of recombination partly gets back to the higher rate of reproduction in microorganisms in comparison with higher organisms. These characteristics render microorganisms highly adaptable to the environment. This may be the reason why microalgae are found in very diverse habitats on Earth from fresh water to saturated salt lakes and the Antarctic sea.Citation39
A new method is presented in for adaptation and genetic screening of microalgae (or other microorganisms) in order to select superior species or strains. Microalgae can be artificially selected to survive in the simulated stressful conditions of the space. Unlike any previous experiments in which different stressors have been simultaneously imposed to select superior microalgae and cyanobacteria,Citation40,Citation41 in this method, stressors can be applied in a stepwise manner. In each stage, a new sublethal stressor can be applied to microalgae cell culture and a couple of cell in a population may survive the challenge, adapt to it and proliferate to enter the other stage while carrying the heritable variations (). The environmental stressors in space or in other extreme situations include but are not limited to microgravity, temperature extremes, high carbon dioxide concentration, heavy metals exposure and high UV radiation. All these stressors can be applied to a microalgae cell culture in a stepwise manner. If this process in continued, a super-species may be selected which will be resistant to all tested stressors. Afterwards, these microalgae can be used for cultivation in BLSS and/or utilized for farming on Moon and Mars. In our opinion, rapid stepwise artificial Darwinian selection of superior microorganisms can open new and interesting avenues for further advances in designing BLSS.
Conclusions
Photosynthetic organisms are considered to be indispensible components of BLSS; however, no known plant may be capable of tolerating such harsh growth conditions. The Earth already possesses a diverse genetic reservoir for us to choose from. Among other green organisms, microalgae are highly tolerant of and adaptable to new stressors. Genetic engineering holds promise for production of superorganisms through introduction of robust enzymes into the photosynthetic machinery of pioneer green organisms. Furthermore, artificial selection can be considered as a tool in screening populations in search for tolerant and adaptable species. The engineered and selected green organisms can then be used in BLSS or employed in terraforming of Mars.
| Abbreviations: | ||
| LSS | = | life support system |
| ALSS | = | advanced life support system |
| BLSS | = | biological or bioregenerative life support system |
| CEEF | = | closed ecology experiment facilities |
| CELSS | = | controlled ecological life support system |
| MELISSA | = | micro-ecological life support system alternative |
Disclosure of Potential Conflicts of interest
The authors declare there are no conflict of interests.
Acknowledgments
The authors are sincerely grateful to Sara Saei for contribution in editing the manuscript.
References
- Roman MC. Life Support Systems Microbial Challenges. 2010. http://ntrs.nasa.gov/search.jsp?R=20100040625
- Barzegari A, Saei AA. Designing probiotics with respect to the native microbiome. Future Microbiol 2012; 7:571 - 5; http://dx.doi.org/10.2217/fmb.12.37; PMID: 22568713
- Saei AA, Barzegari A. The microbiome: the forgotten organ of the astronaut’s body - probiotics beyond terrestrial limits. Future Microbiol 2012; 7:1037 - 46; http://dx.doi.org/10.2217/fmb.12.82; PMID: 22953705
- Brown CS. Programmable Plants: Development of an in planta System for the Remote Monitoring and Control of Plant Function for Life Support. Curr Opin Plant Biol 2000; 3:••• http://www.niac.usra.edu/files/studies/final_report/491Brown.pdf
- Nisbet EG, Sleep NH. The habitat and nature of early life. Nature 2001; 409:1083 - 91; http://dx.doi.org/10.1038/35059210; PMID: 11234022
- Ferl R, Wheeler R, Levine HG, Paul AL. Plants in space. Curr Opin Plant Biol 2002; 5:258 - 63; http://dx.doi.org/10.1016/S1369-5266(02)00254-6; PMID: 11960745
- Tanksley SD, McCouch SR. Seed banks and molecular maps: unlocking genetic potential from the wild. Science 1997; 277:1063 - 6; http://dx.doi.org/10.1126/science.277.5329.1063; PMID: 9262467
- Golueke CG, Oswald WJ. Role of plants in closed systems. Annu Rev Plant Physiol 1964; 15:387 - 408; http://dx.doi.org/10.1146/annurev.pp.15.060164.002131
- Wheeler RM. Plants for human life support in space: from Myers to Mars. Gravit Space Biol 2011;23.
- Monje O, Bingham GE, Carman JG, Campbell WF, Salisbury FB, Eames BK, et al. Canopy photosynthesis and transpiration in microgravity: gas exchange measurements aboard Mir. Adv Space Res 2000; 26:303 - 6; http://dx.doi.org/10.1016/S0273-1177(99)00575-X; PMID: 11543166
- Brown CS, Tibbitts TW, Croxdale JG, Wheeler RM. Potato tuber formation in the spaceflight environment. Life Support Biosph Sci 1997; 4:71 - 6; PMID: 11540455
- Levinskikh MA, Sychev VN, Derendyaeva TA, Signalova OB, Salisbury FB, Campbell WF, et al. Analysis of the spaceflight effects on growth and development of Super Dwarf wheat grown on the Space Station Mir. J Plant Physiol 2000; 156:522 - 9; http://dx.doi.org/10.1016/S0176-1617(00)80168-6; PMID: 11543345
- Croxdale J, Cook M, Tibbitts TW, Brown CS, Wheeler RM. Structure of potato tubers formed during spaceflight. J Exp Bot 1997; 48:2037 - 43; PMID: 11541084
- Salisbury FB, Campbell WF, Carman JG, Bingham GE, Bubenheim DL, Yendler B, et al. Plant growth during the Greenhouse II experiment on the Mir orbital station. Adv Space Res 2003; 31:221 - 7; http://dx.doi.org/10.1016/S0273-1177(02)00744-5; PMID: 12580179
- Stutte GW, Monje O, Goins GD, Tripathy BC. Microgravity effects on thylakoid, single leaf, and whole canopy photosynthesis of dwarf wheat. Planta 2005; 223:46 - 56; http://dx.doi.org/10.1007/s00425-005-0066-2; PMID: 16160842
- Ehleringer JR, Monson RK. Evolutionary and ecological aspects of photosynthetic pathway variation. Annu Rev Ecol Syst 1993; 24:411 - 39; http://dx.doi.org/10.1146/annurev.es.24.110193.002211
- Kozaki A, Takeba G. Photorespiration protects C3 plants from photooxidation. Nature 1996; 384:557 - 60; http://dx.doi.org/10.1038/384557a0
- Dengler NG, Nelson T. Leaf structure and development in C4 plants. In: Sage R and Monson R, eds. C4 Plant Biology. Academic Press, 1999:133-72.
- Osborne CP, Beerling DJ. Nature’s green revolution: the remarkable evolutionary rise of C4 plants. Philos Trans R Soc Lond B Biol Sci 2006; 361:173 - 94; http://dx.doi.org/10.1098/rstb.2005.1737; PMID: 16553316
- Cushman JC. Crassulacean acid metabolism. A plastic photosynthetic adaptation to arid environments. Plant Physiol 2001; 127:1439 - 48; http://dx.doi.org/10.1104/pp.010818; PMID: 11743087
- Raven JA, Edwards D. Roots: evolutionary origins and biogeochemical significance. J Exp Bot 2001; 52:Spec Issue 381 - 401; http://dx.doi.org/10.1093/jexbot/52.suppl_1.381; PMID: 11326045
- Furbank RT, Chitty JA, Jenkins CLD, Taylor WC, Trevanion SJ, von Caemmerer S, et al. Genetic manipulation of key photosynthetic enzymes in the C4 plant Flaveria bidentis. Funct Plant Biol 1997; 24:477 - 85
- Parry M, Madgwick P, Carvalho J, Andralojc P.. Prospects for increasing photosynthesis by overcoming the limitations of Rubisco. Journal of Agricultural Science 2007; 145:31 - 4; http://dx.doi.org/10.1017/S0021859606006666
- Andrews T, Lorimer G. Photorespiration–still unavoidable?. FEBS Lett 1978; 90:1 - 9; http://dx.doi.org/10.1016/0014-5793(78)80286-5
- Long SP, Ainsworth EA, Leakey ADB, Nösberger J, Ort DR. Food for thought: lower-than-expected crop yield stimulation with rising CO2 concentrations. Science 2006; 312:1918 - 21; http://dx.doi.org/10.1126/science.1114722; PMID: 16809532
- Uemura K, Suzuki Y, Shikanai T, Wadano A, Jensen RG, Chmara W, et al. A rapid and sensitive method for determination of relative specificity of RuBisCO from various species by anion-exchange chromatography. Plant Cell Physiol 1996; 37:325 - 31; http://dx.doi.org/10.1093/oxfordjournals.pcp.a028949
- Parry MA, Andralojc PJ, Mitchell RA, Madgwick PJ, Keys AJ. Manipulation of Rubisco: the amount, activity, function and regulation. J Exp Bot 2003; 54:1321 - 33; http://dx.doi.org/10.1093/jxb/erg141; PMID: 12709478
- Gillon J, Yakir D. Naturally low carbonic anhydrase activity in C4 and C3 plants limits discrimination against C18OO during photosynthesis. Plant Cell Environ 2000; 23:903 - 15; http://dx.doi.org/10.1046/j.1365-3040.2000.00597.x
- Wheeler RM, Stutte GW, Mackowiak CL, Yorio NC, Sager JC, Knott WM. Gas exchange rates of potato stands for bioregenerative life support. Adv Space Res 2008; 41:798 - 806; http://dx.doi.org/10.1016/j.asr.2007.07.027
- Edeen M, Dominick J, Barta D, Packham N. “Control of air revitalization using plants: Results of the early human testing initiative phase I test,” SAE Technical Paper 961522. 1996; doi:10.4271/961522.
- Gitelson II, Lisovsky GM. Creation of closed ecological life support systems: Results, critical problems and potentials. Journal of Siberian Federal University. Biology 2008; 1:19 - 39
- Bugbee B, Spanarkel B, Johnson S, Monje O, Koerner G. CO2 crop growth enhancement and toxicity in wheat and rice. Adv Space Res 1994; 14:257 - 67; http://dx.doi.org/10.1016/0273-1177(94)90306-9; PMID: 11540191
- Cockell CS, Schuerger AC, Billi D, Friedmann EI, Panitz C. Effects of a simulated martian UV flux on the cyanobacterium, Chroococcidiopsis sp. 029. Astrobiology 2005; 5:127 - 40; http://dx.doi.org/10.1089/ast.2005.5.127; PMID: 15815164
- Kasting JF, Siefert JL. Life and the evolution of Earth’s atmosphere. Science 2002; 296:1066 - 8; http://dx.doi.org/10.1126/science.1071184; PMID: 12004117
- Saei AA, Ghanbari P, Barzegari A. Haematococcus as a promising cell factory to produce recombinant pharmaceutical proteins. [Online ahead of print] Mol Biol Rep 2012; http://dx.doi.org/10.1007/s11033-012-1861-z; PMID: 22733498
- Barzegari A, Hejazi MA, Hosseinzadeh N, Eslami S, Mehdizadeh Aghdam E, Hejazi MS. Dunaliella as an attractive candidate for molecular farming. Mol Biol Rep 2010; 37:3427 - 30; http://dx.doi.org/10.1007/s11033-009-9933-4; PMID: 19943116
- Lehto KM, Lehto HJ, Kanervo EA. Suitability of different photosynthetic organisms for an extraterrestrial biological life support system. Res Microbiol 2006; 157:69 - 76; http://dx.doi.org/10.1016/j.resmic.2005.07.011; PMID: 16439102
- Antonian AA, Abakumova IA, Meleshko GI, Vlasova TF. [Possibilities of using proteins from unicellular algae in biological life support systems]. [article in Russian] Kosm Biol Aviakosm Med 1985; 19:65 - 9; PMID: 2857798
- Thomas DN, Dieckmann GS. Antarctic Sea ice--a habitat for extremophiles. Science 2002; 295:641 - 4; http://dx.doi.org/10.1126/science.1063391; PMID: 11809961
- Boston P, Todd P, van de Camp J, Northup D, Spilde M. Mars simulation challenge experiments: Microorganisms from natural rock and cave communities. Grav Space Biol 2011; 22:39 - 43
- Billi D, Viaggiu E, Cockell CS, Rabbow E, Horneck G, Onofri S. Damage escape and repair in dried Chroococcidiopsis spp. from hot and cold deserts exposed to simulated space and martian conditions. Astrobiology 2011; 11:65 - 73; http://dx.doi.org/10.1089/ast.2009.0430; PMID: 21294638