Abstract
Adeno-Associated Virus (AAV) has been widely used for in vivo study and preclinical therapy due to its ability to mediate long-term transgene expression, its lack of pathogenicity and low immunogenicity. It has been found that AAV has more than ten serotypes, which each transfect certain types of cells in the viral infected organ. Current methods for purification of different AAV serotypes utilize CsCl or Iodixanol ultrahigh speed density gradient centrifugation, which is expensive and time consuming. We recently developed a simplified method, PEG/(NH4)2SO4 aqueous two phase partitioning, for purification of AAV serotype 2 and 8. The method does not require ultrahigh speed gradient centrifugation or chromatography. Here we further explore the simplified method for purification of other serotypes of AAV, serotype 6 and 9. This simplified method not only can be used to purify serotype 2 and 8, but also serotype 6 and 9, indicating that a variety of AAV serotypes can be purified by this method.
Recombinant adeno-associated viral (AAV) vectors have rapidly advanced to the forefront of gene therapy in recent years. In the past decade, advances with AAV-based vectors have been made by the isolation of several naturally occurring AAV serotypes, together with over 100 AAV variants from different animal species. These isolates are ideally suited to developing human gene therapy vectors due to their diverse tissue tropisms. Currently there is no simple and quick purification method for AAV variants, especially one that is applicable to a variety of different serotypes. CsCl or Iodixanol ultrahigh speed density gradient centrifugation is a traditionally well-known purification method for almost all kinds AAV serotypes, but this method is complex and time consuming. CsCl gradient-based protocols provide flexibility for purification of different serotypes. However, a commonly used first-generation CsCl-based protocol, One-round Ultrahigh Speed CentrifugationCitation1 was found to result in AAV vectors containing large amounts of protein and DNA impurities and low transduction efficiency in vitro and in vivo. New AAV purification methods often reference this first generation CsCl ultrahigh speed centrifugation as a control. PEG-Salt two phase aqueous partitioning, which is easily scaled up, has the ability to handle particulate materials, achieving successful purification of serotype 8 AAV. Klein et al., 2008,Citation2 reported a three-step CsCl gradient ultrahigh speed centrifugation technique with high purity of AAV, but the method is time consuming. Our method, on the other hand, compared with first generation CsCl ultrahigh speed centrifugation, employs a 10%PEG8000–13.2% (NH4)2SO4 aqueous two phase partitioning(ATP), which seems to be a simpler method that saves time().
Figure 1. Time-line comparison of PEG/(NH4)2SO4 two phase aqueous partitioning and first generation CsCl ultrahigh speed centrifugation.
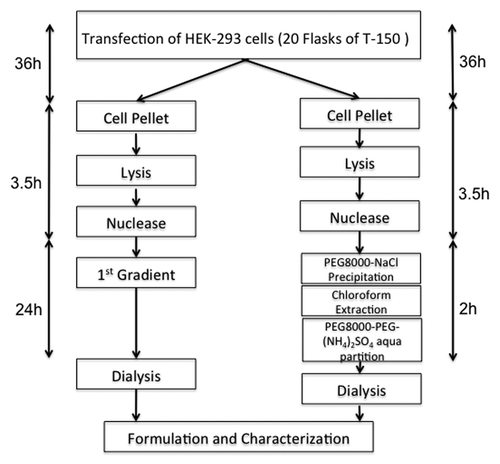
The principle of ATP in protein isolation is that when a mixture of proteins are added to an aqueous two-phase system, each protein distributes between the two phases depending on the protein properties and partition conditions (composition of the system, pH and temperature, etc…). Under appropriate conditions the target protein may be concentrated in the upper phase, while all the other proteins partition into the lower phase resulting in target protein isolation. In our 10%PEG8000–13.2% (NH4)2SO4 aqueous two-phase partitioning method, the polyethylene glycol precipitation step was added for precipitating virus from the cell lysis after DNase and sodium deoxycholate treatments. Before the PEG-Salt aqueous partition processing a step of chloroform extraction was applied to remove the hydrophilic proteins and PEG residues. The partition conditions of 10%PEG8000–13.2% (NH4)2SO4, pH 8.0 at room temperature forces almost all non-viral associated proteins to salt out in precipitation, or else to partition into the top PEG phase and inter-phase, with the virus concentrated in the aqueous phase. In the 10%PEG8000–13.2% (NH4)2SO4 aqueous partition process, since pH volume is critical for protein partitioning, the HEPES buffer has to be kept at pH = 8.0. AAV is more stable in the aqueous phase at alkaline pH than in acid solution, High-efficiency partitioning and viral concentration in the aqueous free of contaminating proteins occurs at alkaline pH.
The advantage of using AAV is that it infects specific tissue cells tropically. We successfully purified AAV serotype 8 from HEK-293 cells by using 10%PEG/13.2%(NH4)2SO4 aqueous two phase partitioning. AAV has been found to have more than ten serotypes and more are likely to be found in the future. Whether the PEG-Salt two-phase aqueous partition method is suitable for purification variants of AAV serotype remains to be seen.
AAV serotype 6 has been recently reported to be tropic for mouse pancreatic ductal cellsCitation3 by using two consecutive caesium chloride (CsCl) gradients, we purified AAV serotype 6 carrying a GFP vector. AAV serotype 9 has also been reported to specifically infect cardiac cells and is widely used to study heart related diseases.Citation4,Citation5 Here we explore the purification of serotypes 6 and 9 of AAV using our 10%PEG/13.2%(NH4)2SO4 aqueous partitioning method.
To create the AAV serotype 6 or 9, the packaging plasmid carries the serotype 6 or 9 AAV rep and cap genes. While the helper plasmid carries the adenovirus helper functions. Both plasmids are then co-transfected with pAAV-CMV-ZsGreen plasmid, using PEI reagent to transfect HEK293 cells. We then used the 10%PEG/13.2%(NH4)2SO4 aqueous partitioning protocol to purify AAV6 and AAV9 viruses.
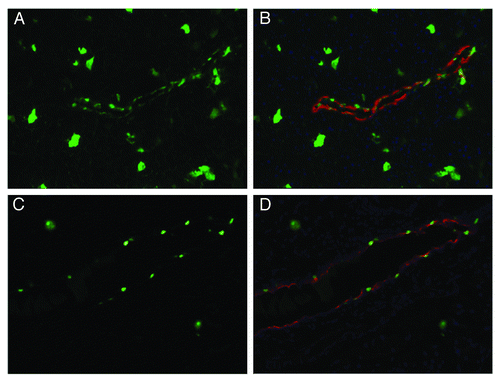
For assessing the adequacy of viral infection after 10%PEG/13.2%(NH4)2SO4 aqueous partitioning, a common bile ductal injection technique using purified AAV6-CMV-ZsGreen (AAV6-GFP) was employed.Citation6 We found a better transduction rate compared with the first generation CsCl gradients ultrahigh speed centrifugation purified virus ().
Figure 2. Comparing infection rate using the simplified PEG/(NH4)2SO4 two phase aqueous partitioning method and CsCl first generation method. Animal procedures and virus injection were preformed with prior approval of the University of Pittsburgh Institutional Animal Care and Use Committee (protocol number: 1103211). The anesthetized 10 weeks old CD1 mice (n = 3) were injected with 100 μl of AAV6-GFP (titer 2.3 × 1012 GCP/ml) through the common bile duct. A clip was placed on the bile duct near the hilum of liver to clamp the hepatic duct, leading to specific perfusion of the pancreatic ducts. Pancreatic ducts infected with 10%PEG/13.2%(NH4)2SO4 aqueous partitioning purified AAV6-GFP (A) or with first generation CsCl gradients ultrahigh speed centrifugation purified virus (C). (B and D) are merged counterstained with DBA for ducts (red), DAPI(blue) and GFP.
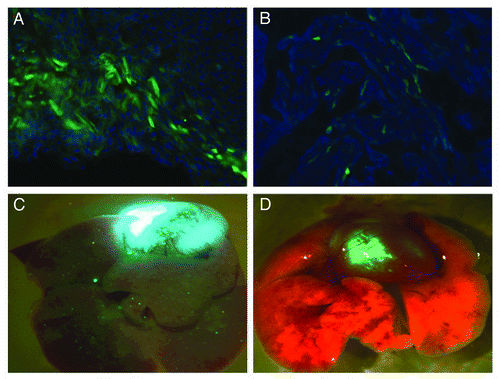
GFP transduction after 10%PEG/13.2%(NH4)2SO4 aqueous partitioning in mouse embryos was achieved using purified AAV9-CMV-ZsGreen (AAV9-GFP), which was delivered using an transuterine intracardiac injection system.Citation7 Similarly, the results showed better transduction rates compared with first generation CsCl gradients ultrahigh speed centrifugation purified virus ().
Figure 3. Comparing the infection rate of AAV9-GFP in mouse embryos using the simplified PEG/(NH4)2SO4 two phase aqueous partitioning method or CsCl first generation method. Mouse embryonic cardiomyocytes had a higher transfection rate with 10%PEG/13.2%(NH4)2SO4 aqueous partitioning purified AAV9-GFP (A) than first generation CsCl gradients ultrahigh speed centrifugation purified virus (B); dissected heart and lung gross view in (C and D) respectively. E13.5 CD1 mouse embryos (n = 5) were injected with 5ul of AAV9-GFP (titer 2.3 × 1012 GCP/ml) through intracardiac injection with either 10%PEG/13.2%(NH4)2SO4 aqueous partitioning purified virus or first generation CsCl gradient ultrahigh speed centrifugation purified AAV9-GFP virus. Briefly, the pregnant CD1 mice were anesthetized and subjected to a laparotomy, the uterus exposed and then a fenestrated dish was placed over the mouse and a single embryo brought through the fenestration. The ultrasound microscope brobe is used to guide the injection apparatus. A glass needle is used to inject the heart with different methods purified AAV-CMV-GFP serotype 9 virus.Citation7 The post natal 5 d CD1 mice were harvested, sectioned and the cardiomyocytes were counterstained with DAPI (blue).
The use of cesium chloride (CsCl) gradient-based protocols provides the flexibility for purification of different serotypes. However, a commonly used first-generation CsCl-based protocol was found to result in AAV vectors containing large amounts of protein and DNA impurities and low transduction efficiency in vitro and in vivo. The first-generation CsCl-based protocol, which employs one round CsCl ultrahigh speed centrifugation purified methodCitation8 was used as a control approach in several publicationsCitation2 and ref. Citation9. CsCl ultrahigh speed centrifugation is a time consuming and expensive equipment dependent on the speed of the centrifuge and rotor used. Our simplified PEG/(NH4)2SO4 method achieves a higher quality with less time.
To achieve higher purity, researchers have used the three-step CsCl gradients purified AAVCitation2 compared with one round CsCl ultrahigh speed centrifugation method but is much more time consuming. We on the other hand used a one-step CsCl gradient centrifugation as a control in the paper because the method is addressing an approach with rapid, low-budget and simplified steps to reach high enough quality in preclinical animal experiment.
Our results demonstrate that this simplified and relatively quick method of using ATP partitioning 10%PEG/13.2%(NH4)2SO4 to purify the AAV is applicable for purifying serotypes 2, 6, 8 and 9 and likely can also be applied for purification of other variants and serotypes of AAV.
Disclosure of Potential Conflicts of Interest
The authors declare no competing financial interests.
Acknowledgments
We would like to thank Lauren Brink for her assistant with animal work.
Author contributions: P.G. and X.X. designed and performed the experiments, analyzed data and wrote the manuscript. J.P. performed embryonic intra cardiac microinjection. Y.G. viewed and corrected the manuscript. G.G. viewed and finalized the manuscript.
References
- Rose JA, Hoggan MD, Shatkin AJ. Nucleic acid from an adeno-associated virus: chemical and physical studies. Proc Natl Acad Sci U S A 1966; 56:86 - 92; http://dx.doi.org/10.1073/pnas.56.1.86; PMID: 5229859
- Klein RL, Dayton RD, Tatom JB, Henderson KM, Henning PP. AAV8, 9, Rh10, Rh43 vector gene transfer in the rat brain: effects of serotype, promoter and purification method. Mol Ther 2008; 16:89 - 96; http://dx.doi.org/10.1038/sj.mt.6300331; PMID: 17955025
- Jimenez V, Ayuso E, Mallol C, Agudo J, Casellas A, Obach M, et al. In vivo genetic engineering of murine pancreatic beta cells mediated by single-stranded adeno-associated viral vectors of serotypes 6, 8 and 9. Diabetologia 2011; 54:1075 - 86; http://dx.doi.org/10.1007/s00125-011-2070-3; PMID: 21311856
- Bish LT, Morine K, Sleeper MM, Sanmiguel J, Wu D, Gao G, et al. Adeno-associated virus (AAV) serotype 9 provides global cardiac gene transfer superior to AAV1, AAV6, AAV7 and AAV8 in the mouse and rat. Hum Gene Ther 2008; 19:1359 - 68; http://dx.doi.org/10.1089/hum.2008.123; PMID: 18795839
- Prasad KM, Xu Y, Yang Z, Acton ST, French BA. Robust cardiomyocyte-specific gene expression following systemic injection of AAV: in vivo gene delivery follows a Poisson distribution. Gene Ther 2011; 18:43 - 52; http://dx.doi.org/10.1038/gt.2010.105; PMID: 20703310
- Perides G, van Acker GJ, Laukkarinen JM, Steer ML. Experimental acute biliary pancreatitis induced by retrograde infusion of bile acids into the mouse pancreatic duct. Nat Protoc 2010; 5:335 - 41; http://dx.doi.org/10.1038/nprot.2009.243; PMID: 20134432
- Shah SR, Esni F, Jakub A, Paredes J, Lath N, Malek M, et al. Embryonic mouse blood flow and oxygen correlate with early pancreatic differentiation. Dev Biol 2011; 349:342 - 9; http://dx.doi.org/10.1016/j.ydbio.2010.10.033; PMID: 21050843
- Walz C, Schlehofer JR, Flentje M, Rudat V, zur Hausen H. Adeno-associated virus sensitizes HeLa cell tumors to gamma rays. J Virol 1992; 66:5651 - 7; PMID: 1323717
- Ayuso E, Mingozzi F, Montane J, Leon X, Anguela XM, Haurigot V, et al. High AAV vector purity results in serotype- and tissue-independent enhancement of transduction efficiency. Gene Ther 2010; 17:503 - 10; http://dx.doi.org/10.1038/gt.2009.157; PMID: 19956269