Abstract
The baculovirus/insect cell system has proven to be a powerful tool for the expression of eukaryotic proteins. Therapeutics, especially in the field of vaccinology, are often composed of several different protein subunits. Conventional baculoviral expression schemes largely lack efficient strategies for simultaneous multi-gene expression. The MultiBac technology which is based on an engineered genome of Autographa californica nuclear polyhedrosis virus in combination with specially designed transfer vectors is an elegant way for flexible generation of multi-subunit proteins in insect cells. Yet, the glycosylation pattern of insect cell-derived products is not favorable for many applications. Therefore, a modified version of MultiBac, SweetBac, was generated allowing for a flexible glycosylation of target proteins in insect cells. Beyond the SweetBac technology MultiBac can further be designed for bridging the gap between cell engineering and transient modulation of host genes for improved and product tailored expression of recombinant proteins.
Introduction
The great potential of the baculovirus insect cell system for the production of therapeutically active proteins has regained enormous interest by the market entry of the human papilloma virus vaccine Cervarix®Citation1 in 2007. Nowadays, a variety of baculoviral expression systems and suitable insect cell lines ranging from the widely popular Spodoptera frugiperda Sf9 and Sf21 cell lines, to the Trichoplusia ni cell lines BTI-TN5B1–4 “High Five” and BTI-Tnao38, are available.Citation2-Citation5 To date, hundreds of eukaryotic proteins, mainly composed of single subunits, have successfully been produced in insect cells.Citation6 However, therapeutics, especially in the field of vaccinology are often composed of numerous different protein subunits. The recent development of production platforms for influenza A virus-like particlesCitation7-Citation9 takes advantage of the fact that multisubunit particles are much more efficient in eliciting proper immune response as compared with single protein vaccines. Additionally, the possibility of multigene expression can be extremely beneficial, as it allows for co-producing key factors that help to increase the quality of the final product in any desired way, e.g., by target-tailored glycosylation. Yet, the classical baculoviral expression system lacks efficient strategies for the simultaneous expression of many proteins within one cell. There are a small number of commercially available plasmids that enable the construction of multigene expression constructs. However, these are inconvenient and cumbersome in handling due to their already large size and lack of flexibility in their design. A more flexible approach is to co-infect insect cells with several recombinant baculoviruses at the same time, each carrying one or two expression cassettes. This strategy works reasonably well for small-scale experiments, but scale-up is typically intractable and inefficient. Moreover, statistics dictate that the chances of getting all viral clones into one cell decreases with their number. In order to overcome these problems, a flexible baculoviral multi-gene expression system was developed—MultiBac.Citation10-Citation13 So far, the MultiBac system has been primarily used for the expression of heterologous multiprotein complexes dedicated for structural characterization. Other studies demonstrated the use of MultiBac for the generation of virus like particles (VLPs). Such particles consist of proteins that form a viral outer shell and its envelope, without including the genomic elements required for replication. L1 structural protein of human papillomavirus (HPV) subtypes 16 and 18 self assembles into VLPs, which are the basis for CervarixTM. The production of these VLPs using a conventional baculovirus is approved at an industrial scale, but the same setup cannot be used for several other viral subtypes because of very low yields. However, based on the MultiBac technology, yields for several HPV subtypes could be drastically increased (up to 40-fold).Citation14
Baculovirus Expression Systems
The original method for the generation of recombinant baculovirus employed homologous recombination of a transfer vector with the circular wildtype baculovirus DNA in insect cells. The process was very inefficient and required extensive plaque purification for isolating single clones. Ever since, numerous attempts have been made to reduce time span, improve efficacy and user-friendliness of the baculovirus expression systems. Furthermore, virus genome engineering to enhance yield and quality of the heterologous protein and transfer vector design for multigene-expression became an important issue (see ).
Table 1. Comparison of Baculovirus expression systems
BacPAK 6 / BaculoGold
Efficacy of the homologous recombination was improved by engineering three copies of the restriction site Bsu36I into the baculovirus DNA backbone. Upon triple-digestion the linearized genome lacks gene orf1629, which is essential for virus replication.Citation15 Viability is restored upon recombination with a transfer vector providing orf1629 along with the recombinant gene. An array of transfer vectors is available that utilize the baculovirus late and very late promoters, provide purification tags or fusion to the gp64 signal peptide for improved secretion. A maximum of four genes can be expressed form a quadruple promoter vector that contains two copies of p10 and pH promoter each. However, the multiple cloning sites (MCS) only offer a modest number of restriction sites that constrains flexibility in the cloning procedure.
BacVector
The BacVector system is based on a traditional Triple Cut Virus DNA. The BacVector-2000 has a deletion in five non-essential genes to improve target gene expression by decreasing the metabolic burden for the insect cells. Furthermore, two additional genes v-cath (cathepsin)Citation16 and chiA (chitinase A)Citation17 have been deleted from the BacVector-3000. Deletion of chiA gene was shown to enhance secretion and membrane-targeted protein production, since this protein would otherwise accumulate in the secretory pathway. By deleting the v-cath gene, proteolysis and degradation of susceptible target proteins is prevented.Citation18 For cloning the gene of interest into the virus backbone, a number of transfer vectors are available. However, only up to four genes can be simultaneously co-expressed from the available quadruple vector that contains 2 copies of pH and p10 promoters.
Bac-to-Bac
Nowadays, several systems where bacterial artificial chromosomes (BACs) are incorporated into the baculovirus genome are available. The BAC replicon allows the viral DNA to be maintained and amplified in bacterial hosts as bacmids, in a similar manner to large plasmids. Among these systems, the Bac-to-Bac system is most commoly used. It is based on in vivo bacterial site-specific transposition of an expression cassette from a transfer vector into the bacmid. Expression cassettes on the transfer vectors are flanked by the left and right arms of the bacterial Tn7 transposon. As the attachment site for the transposon is located at the N-terminus of the lacZα gene, insertion of the gene of interest into the bacmid causes disruption of the lacZ reading frame resulting in a white phenotype.Citation19 The method is convenient as bacterial cells are utilized for the manipulatation and identification of recombinant baculovirus DNA. As the isolated recombinant bacmid is not contaminated with wildtype viral DNA, there is no need for tedious plaque purification. A maximum of two genes can be concomitantly expressed from the dual vector under control of the very late promoters pH and p10.
flashBAC
The flashBAC system combines the bacmid technology with in vivo recombination in insect cells. It is based on a modified AcNPV backbone that has a deletion in the essential gene orf1629 and contains a bacterial artificial chromosome (BAC) at the polyhedrin gene locus replacing polyhedrin gene.Citation20 The partial deletion of orf1629 renders the virus inactive and prevents replication of any non-recombinant parental virus in insect cells. Homologous recombination between a transfer and the flashBAC backbone restores the function of the essential gene and allows for virus replication. As non-recombinant virus is unable to replicate, plaque purification is obsolete. The gene of interest is inserted under control of the pH promoter with the concomitant removal of the BAC sequence. This is a specific property of the flashBAC technology (compared with Bac-to-Bac) and contributes to genomic stability of the DNA within insect cells. The flashBACGOLD backbone DNA shows improved efficacy of the secretory pathway and yield as well as reduced proteolysis by deletion of chiA and v-cath. flashBACULTRA is the latest development of the flashBAC technology and carries three more deletions of virus non-essential genes (p10, p26 and p74) for improved yield and quality of recombinant proteins.Citation21 A maximum of two heterologous genes can be expressed in parallel from the baculovirus very late promoters (pH and p10) with the dual promoter vector. However, the system does not provide any vectors for multigene expression.
MultiBac
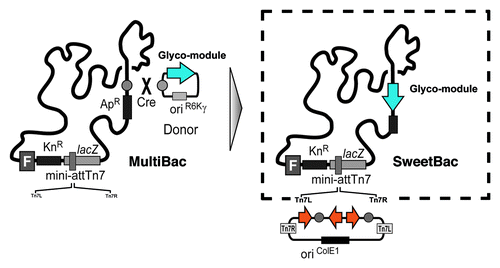
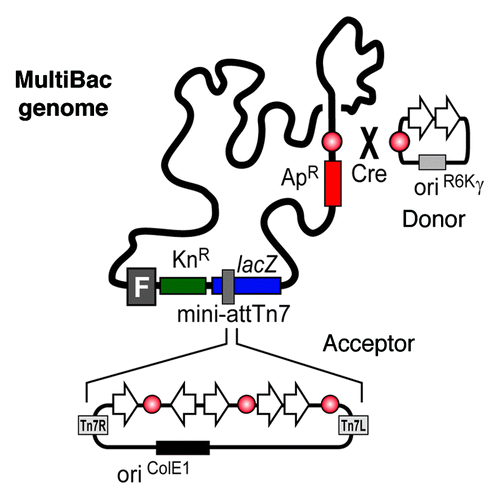
The MultiBac systemCitation10-Citation13 is based on the combination of an engineered viral bacmid, comparable to Bac-to-Bac, and specially designed transfer vectors, so called “acceptors” and “donors.” Both classes of vectors contain expression cassettes consisting of the baculoviral polyhedron (pH) and p10 promoters and eukaryotic polyadenylation signals. The plasmids are further equipped with resistance markers, a short imperfect inverted repeat (LoxP) and an origin of replication (). In case of acceptors the origin is designed for propagation in standard cloning strains of E. coli, whereas donors harbor a conditional origin of replication (derived from phage R6Kγ). Donors can therefore only be propagated in E. coli strains expressing the π-protein encoded by the pir gene. The vector system provides two synergistic features that enable rapid, flexible and efficient generation of multigene constructs. First, expression cassettes are flanked by homing endonucelases allowing for an easy assembly of multiple cassettes on one vector backbone (). By means of a second strategy, one or several donor vectors, containing one or several genes of interest each, can be fused to a single acceptor vector with further genes of interest using the LoxP sites in a Cre-recombinase mediated reaction (). The R6Kγ ori of donor vectors is a crucial feature within this strategy, because only vectors fused to an acceptor are able to survive in a pir-negative environment which is common to all generally used cloning strains.
Figure 1. The MultiBac vector system consists of an array of small synthetic DNA plasmids called acceptors and donors (A). Both classes contain a dual expression cassette (polh and p10) with eukaryotic polyadenylation signals (SV40 and HSVtk). Propagation of acceptors is driven by a regular origin of replication (oriColE1), whereas donors have a conditional origin (oriR6Kγ) that requires special bacterial strains for replication. For selection in E.coli, acceptors contain a gentamycin resistance gene (GnR) and donors one antibiotic marker (AbR). For introduction in the MultiBac viral genome all vectors are equipped with a LoxP site and acceptors additionally with Tn7 transposition sites (Tn7L, Tn7R). Vectors are designed for an easy multiplexing of expression cassettes by the presence of specially designed restriction sites (B). Multigene constructs can futher be generated by fusing several donor vectors with one acceptor vector (C).
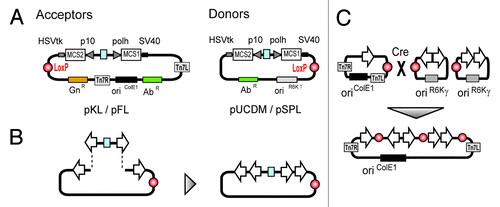
The insertion of genes into the MultiBac genome is possible by two independent mechanisms. First, multigene constructs containing an acceptor can be transformed in DH10MultiBac bacterial strains where co-produced Tn7 transposase inserts the expression cassettes into the Tn7 attachment site present on the viral genome. By a second strategy, genes present on a Donor can be inserted in the distal LoxP site engineered into the viral genome in an in vivo Cre mediated reaction (). For enhancing product quality and stability v-cath and chiA genes have further been inactivated in the MultiBac genome.
Figure 2. Multigene constructs based on acceptor vectors can be inserted in the MultiBac genome via the Tn7 transposition site. Within a second strategy expression cassettes can be inserted in the viral LoxP site in a Cre mediated reaction.
The strength of MultiBac is its capability of expressing complex multi-protein subunits from one recombinant baculovirus. The specially designed cloning strategy via multiple vectors provides flexibility in construct generation and multigene assembly. Subunits can be cloned in a modular manner into separate vectors and mixed and shuffled as per requirement to yield individual expression ensembles.
MultiBac: A Useful Tool for the Expression of Therapeutics
Producing influenza A vaccines is one of the most challenging tasks in vaccine design and process engineering, as the high variability within strains results in altered infectivity, propagation and immunogenic efficacy of the produced vaccine, rendering adaptation of process parameters and testing of the product a necessary requirement every year. As an alternative to whole virus vaccines, the production of virus like particles (VLPs) has shown to be feasible in terms of immunogenicity (Cerverix),Citation1 bearing the advantage of avoiding differences in the dynamics of the infectious cycle. Influenza A VLPs have been previously produced in various expression systems such as plants, mammalian and insect cells.Citation8,Citation22,Citation23 First reports came from Latham and Galarza in 2001 who expressed four structural influenza proteins, hemagglutinin (HA), neuraminidase (NA), maxtrix protein 1 (M1) and M2 simultaneously in Sf9 cells.Citation24 Influenza VLPs can be produced by co-expression of HA, NA, M1 and M2, or HA, NA and M1, or even only HA and M1, respectively.Citation22 To date, VLPs of influenza A subtypes H1, H3, H5, H7, H9 and influenza B have successfully been produced in insect cells. Almost all were tested in mice or ferrets and induced immune responses when administered intranasally, intraperitoneally or intramuscularly.Citation24-Citation27 So far the production of VLPs has been performed using conventional co-infection strategies. Our recent work has evaluated the use of MultiBac for enhancing flexibility and efficiency in the process of influenza VLP generation. Different subtypes of viral HA have therefore individually been cloned into the donor vector pUCDM, while different subtypes of viral M1 were cloned into the acceptor vector pKL. Several combinations of HA and M1 have been tested for optimal VLP generation by fusing specific pUCDM and pKL vectors using the LoxP site in a cre-mediated reaction (). The multigene construct was then integrated into the Tn7 site of the MultiBac baculoviral genome (). The MultiBac technology was shown to enhance the speed and efficiency of VLP generation and, thus, is highly convenient for testing many different combinations of HA and M1 in a reasonable time frame.
Insect Cells and Their Glycosylation: Possible Impact on Therapeutics
A major limitation regarding the production of therapeutic proteins in insect cell lines is the lack of complex type N-glycans. N-glycans found on insect cell-expressed proteins are mainly of a high mannose type or have non-fucosylated and core-fucosylated paucimannosidic structures.Citation28 Especially the core α1,3-linked fucose, which is known to be immunogenic, is not present in mammalian cells, but is a very common modification in invertebrates. On the other hand, the immunogenic potential of α1,3-linked fucose could have an adjuvant effect for vaccine preparations.
For example, influenza surface proteins HA and NA both are specifically glycosylated for a variety of important functions including fusion activity, antigenic properties, infectivity and virus release.Citation29 HA undergoes host-cell dependent glycosylation that is crucial for proper folding of the molecule during infection. All HA molecules are generally glycosylated to varying degrees and it is known that carbohydrate addition can have both positive and negative effects on the virus. The glycosylation of influenza HA is further critical for immune recognition and therefore, has a major influence on the efficacy of vaccine preparations. Carbohydrate positioned around the antigenic sites of the globular head of HA for example can mask antigenic sites from immune recognition.Citation29 Additionally, glycosylation sites in the highly conserved stalk domain of the HA seem to be conserved as well and might shield this functionally important domain from effective immune responses. In order to validate the actual impact of insect cell-produced vaccines on the immune system, and to test which types of glycosylation are beneficial and which are adverse, a flexible tool for specifically targeted glycoengineering is required.
Making It Sweet: SweetBac
In the past, several approaches have served to improve N-linked glycosylation in lepidopteran insect cell lines by expressing additional glycozymes. Transient approaches were mainly based on the co-infection of insect cells with viruses expressing the target gene together with viruses encoding glycozymes.Citation30 A common problem employing such a setup is the low efficiency of co-infections. Another approach for modifying the N-linked glycosylation pathways in insects is the stable integration of glycozyme open reading frames in the genome of a host cell. Using this approach several transgenic insect cell lines have been generated successfully, most of them based on Sf9 cells.Citation31,Citation32 The drawback of this strategy is a possible metabolic overload for the transgenic insect cell line, leading to reduced growth characteristics and long-term instability as well as reduced yields of recombinantly produced proteins. Furthermore, the modified N-glycosylation patterns might influence the functionality of cellular proteins and have a wider impact on the robustness of the system. To overcome these problems, a recent study has reported the generation of a new transgenic Sf9 cell line with an inducible mammalian-like protein N-glycosylation machinery.Citation33 However, all these approaches are based on Sf9 cells, but for the expression of secreted proteins, cell lines derived from Trichoplusia ni have been demonstrated to produce significantly higher amounts in many cases.Citation9,Citation34,Citation35
The recently developed SweetBac system,Citation35 a platform for the expression of mammalianised proteins in insect cells that is based on MultiBac, might serve to solve the problems stated above (a schematic representation of SweetBac is shown in ). In its first embodiment, a so-called glyco-module, consisting of open reading frames coding for the Caenorhabditis elegans N-acetylglucosaminyltransferase II and the bovine β1,4-galactosyltransferase I, controlled by polyhedrin and p10 promoters respectively, was introduced in the LoxP site of the MultiBac baculoviral genome. The resulting SweetBac backbone was subsequently used as a shuttle vector for the expression of human HIV anti-gp41 antibody 3D6 integrated in the standard Tn7 site. Antibody molecules are ideal targets for testing the functionality of SweetBac because of their glycan-dependency and routine methods for analysis. Simultaneous expression of target genes and glycosyltransferases in most commonly used insect cell lines gave an overall expression rate comparable to that of transient expression in mammalian cells. Especially the results for the recently established Tnao38 cell line must be highlighted, because yields of 30 μg/ml cell culture supernatant were reported.Citation35 The presence of terminal galactose residues on SweetBac expressed antibodies was confirmed by analyzing PNGase A released N-glycans with MALDI-TOF-MS. The more complex type N-glycan structures had no influence on antibodies target binding ability, but caused a significantly enhanced binding to human Fc gamma receptor I (CD64). This indicates an increased ability to exert effector functions like antibody-dependent cellular cytotoxicity (ADCC).
Cell Modulation
Cell engineering is often the only choice for improving the quality or the yield of a product in animal cells or also in yeast and bacterial cells. Enzymes, chaperones and other factors that improve recombinant protein production may be stably integrated into the host’s genome. Also knockout mutants or gene silencing can be achieved by this strategy. In contrast to stable cell lines, expression systems based on a viral infection cycle are transient and product yield and quality varies with multiplicity of infection, robustness of the cells, and interaction of viral and cellular proteins, e.g., initiation of apoptosis, unfolded protein response and other factors. Since an engineered cell mostly suffers from a loss in robustness, especially when it comes to additionally expressed foreign glycozymes, simultaneous co-expression of modulating enzymes during the production process appears to be an attractive alternative. Not only additional overexpression of certain co-factors, but also individual up- and downregulation of any host gene becomes possible. Instead of cell engineering, we suggest to modulate gene expression by introducing arrays of relevant proteins into baculovirus based on the MultiBac system. Besides higher flexibility, the cells do not suffer from an additional metabolic burden when uninfected, there is no risk of cellular instabilities, and the expression level of the co-factors corresponds to the number of infectious particles per cell. Thus, for example, higher multiplicities of infection automatically provide a higher dosage of e.g., chaperones or glycosyltransferases. The system is not limited to the expression of beneficial proteins; knock-downs by anti-sense RNAs may serve to diminish e.g., undesired enzymatic activities. Yet, whole genome sequences or the full sets of cDNAs that allow identification of relevant target genes are not available for these insect cell lines. However, projects addressing these issues are on their way.
Next generations of the SweetBac platform could comprise a set of viral backbones containing glyco-modules or other genes that are involved in important pathways, enabling a large variety of cell-modulation approaches. Thus, the MultiBac technology, together with increased knowledge about genome structure, gene function and regulatory pathways of insect cells brings enormous advantages to baculovirus based expression systems in terms of flexible product design and fine-tuning of the production process.
| Abbreviations: | ||
| VLPs | = | virus-like particles |
| HA | = | hemagglutinin |
| NA | = | neuraminidase |
| M1 | = | matrix protein 1 |
| MALDI-TOF-MS | = | matrix-assisted laser desorption ionization mass spectrometry |
References
- Schiller JT, Castellsagué X, Villa LL, Hildesheim A. An update of prophylactic human papillomavirus L1 virus-like particle vaccine clinical trial results. Vaccine 2008; 26:Suppl 10 K53 - 61; http://dx.doi.org/10.1016/j.vaccine.2008.06.002; PMID: 18847557
- Hashimoto Y, Zhang S, Blissard GW. Ao38, a new cell line from eggs of the black witch moth, Ascalapha odorata (Lepidoptera: Noctuidae), is permissive for AcMNPV infection and produces high levels of recombinant proteins. BMC Biotechnol 2010; 10:50; http://dx.doi.org/10.1186/1472-6750-10-50; PMID: 20602790
- Summers MD, Smith GE. A manual of methods for baculovirus vectors and insect cell culture procedures. Texas Agricultural Experiment Station Bulletin 1987; 1555
- Vaughn JL, Goodwin RH, Tompkins GJ, McCawley P. The establishment of two cell lines from the insect Spodoptera frugiperda (Lepidoptera; Noctuidae). In Vitro 1977; 13:213 - 7; http://dx.doi.org/10.1007/BF02615077; PMID: 68913
- Wickham TJ, Nemerow GR. Optimization of growth methods and recombinant protein production in BTI-Tn-5B1-4 insect cells using the baculovirus expression system. Biotechnol Prog 1993; 9:25 - 30; http://dx.doi.org/10.1021/bp00019a004; PMID: 7764044
- Kost TA, Condreay JP, Jarvis DL. Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat Biotechnol 2005; 23:567 - 75; http://dx.doi.org/10.1038/nbt1095; PMID: 15877075
- Krammer F, Grabherr R. Alternative influenza vaccines made by insect cells. Trends Mol Med 2010; 16:313 - 20; http://dx.doi.org/10.1016/j.molmed.2010.05.002; PMID: 20570562
- Krammer F, Nakowitsch S, Messner P, Palmberger D, Ferko B, Grabherr R. Swine-origin pandemic H1N1 influenza virus-like particles produced in insect cells induce hemagglutination inhibiting antibodies in BALB/c mice. Biotechnol J 2010; 5:17 - 23; http://dx.doi.org/10.1002/biot.200900267; PMID: 20041443
- Krammer F, Schinko T, Palmberger D, Tauer C, Messner P, Grabherr R. Trichoplusia ni cells (High Five) are highly efficient for the production of influenza A virus-like particles: a comparison of two insect cell lines as production platforms for influenza vaccines. Mol Biotechnol 2010; 45:226 - 34; http://dx.doi.org/10.1007/s12033-010-9268-3; PMID: 20300881
- Berger I, Fitzgerald DJ, Richmond TJ. Baculovirus expression system for heterologous multiprotein complexes. Nat Biotechnol 2004; 22:1583 - 7; http://dx.doi.org/10.1038/nbt1036; PMID: 15568020
- Bieniossek C, Richmond TJ, Berger I. MultiBac: multigene baculovirus-based eukaryotic protein complex production. Curr Protoc Protein Sci 2008; Chapter 5:Unit 5.20.
- Fitzgerald DJ, Berger P, Schaffitzel C, Yamada K, Richmond TJ, Berger I. Protein complex expression by using multigene baculoviral vectors. Nat Methods 2006; 3:1021 - 32; http://dx.doi.org/10.1038/nmeth983; PMID: 17117155
- Trowitzsch S, Bieniossek C, Nie Y, Garzoni F, Berger I. New baculovirus expression tools for recombinant protein complex production. J Struct Biol 2010; 172:45 - 54; http://dx.doi.org/10.1016/j.jsb.2010.02.010; PMID: 20178849
- Senger T, Schädlich L, Gissmann L, Müller M. Enhanced papillomavirus-like particle production in insect cells. Virology 2009; 388:344 - 53; http://dx.doi.org/10.1016/j.virol.2009.04.004; PMID: 19409593
- Possee RD, Sun TP, Howard SC, Ayres MD, Hill-Perkins M, Gearing KL. Nucleotide sequence of the Autographa californica nuclear polyhedrosis 9.4 kbp EcoRI-I and -R (polyhedrin gene) region. Virology 1991; 185:229 - 41; http://dx.doi.org/10.1016/0042-6822(91)90770-C; PMID: 1926775
- Hom LG, Volkman LE. Autographa californica M nucleopolyhedrovirus chiA is required for processing of V-CATH. Virology 2000; 277:178 - 83; http://dx.doi.org/10.1006/viro.2000.0586; PMID: 11062048
- Slack JM, Kuzio J, Faulkner P. Characterization of v-cath, a cathepsin L-like proteinase expressed by the baculovirus Autographa californica multiple nuclear polyhedrosis virus. J Gen Virol 1995; 76:1091 - 8; http://dx.doi.org/10.1099/0022-1317-76-5-1091; PMID: 7730794
- Hitchman RB, Possee RD, Crombie AT, Chambers A, Ho K, Siaterli E, et al. Genetic modification of a baculovirus vector for increased expression in insect cells. Cell Biol Toxicol 2010; 26:57 - 68; http://dx.doi.org/10.1007/s10565-009-9133-y; PMID: 19655260
- Luckow VA. Baculovirus systems for the expression of human gene products. Curr Opin Biotechnol 1993; 4:564 - 72; http://dx.doi.org/10.1016/0958-1669(93)90078-B; PMID: 7764207
- Possee RD, Hitchman RB, Richards KS, Mann SG, Siaterli E, Nixon CP, et al. Generation of baculovirus vectors for the high-throughput production of proteins in insect cells. Biotechnol Bioeng 2008; 101:1115 - 22; http://dx.doi.org/10.1002/bit.22002; PMID: 18781697
- Hitchman RB, Possee RD, Siaterli E, Richards KS, Clayton AJ, Bird LE, et al. Improved expression of secreted and membrane-targeted proteins in insect cells. Biotechnol Appl Biochem 2010; 56:85 - 93; http://dx.doi.org/10.1042/BA20090130; PMID: 20441568
- Kang SM, Song JM, Quan FS, Compans RW. Influenza vaccines based on virus-like particles. Virus Res 2009; 143:140 - 6; http://dx.doi.org/10.1016/j.virusres.2009.04.005; PMID: 19374929
- Krammer F, Schinko T, Palmberger D, Tauer C, Messner P, Grabherr R. Trichoplusia ni cells (High Five) are highly efficient for the production of influenza A virus-like particles: a comparison of two insect cell lines as production platforms for influenza vaccines. Mol Biotechnol 2010; 45:226 - 34; http://dx.doi.org/10.1007/s12033-010-9268-3; PMID: 20300881
- Latham T, Galarza JM. Formation of wild-type and chimeric influenza virus-like particles following simultaneous expression of only four structural proteins. J Virol 2001; 75:6154 - 65; http://dx.doi.org/10.1128/JVI.75.13.6154-6165.2001; PMID: 11390617
- Galarza JM, Latham T, Cupo A. Virus-like particle (VLP) vaccine conferred complete protection against a lethal influenza virus challenge. Viral Immunol 2005; 18:244 - 51; http://dx.doi.org/10.1089/vim.2005.18.244; PMID: 15802970
- Pushko P, Pearce MB, Ahmad A, Tretyakova I, Smith G, Belser JA, et al. Influenza virus-like particle can accommodate multiple subtypes of hemagglutinin and protect from multiple influenza types and subtypes. Vaccine 2011; 29:5911 - 8; http://dx.doi.org/10.1016/j.vaccine.2011.06.068; PMID: 21723354
- Ross TM, Mahmood K, Crevar CJ, Schneider-Ohrum K, Heaton PM, Bright RA. A trivalent virus-like particle vaccine elicits protective immune responses against seasonal influenza strains in mice and ferrets. PLoS One 2009; 4:e6032; http://dx.doi.org/10.1371/journal.pone.0006032; PMID: 19554101
- Altmann F, Staudacher E, Wilson IB, März L. Insect cells as hosts for the expression of recombinant glycoproteins. Glycoconj J 1999; 16:109 - 23; http://dx.doi.org/10.1023/A:1026488408951; PMID: 10612411
- Schulze IT. Effects of glycosylation on the properties and functions of influenza virus hemagglutinin. J Infect Dis 1997; 176:Suppl 1 S24 - 8; http://dx.doi.org/10.1086/514170; PMID: 9240690
- Jarvis DL, Finn EE. Modifying the insect cell N-glycosylation pathway with immediate early baculovirus expression vectors. Nat Biotechnol 1996; 14:1288 - 92; http://dx.doi.org/10.1038/nbt1096-1288; PMID: 9631095
- Aumiller JJ, Hollister JR, Jarvis DL. A transgenic insect cell line engineered to produce CMP-sialic acid and sialylated glycoproteins. Glycobiology 2003; 13:497 - 507; http://dx.doi.org/10.1093/glycob/cwg051; PMID: 12626399
- Hollister J, Grabenhorst E, Nimtz M, Conradt H, Jarvis DL. Engineering the protein N-glycosylation pathway in insect cells for production of biantennary, complex N-glycans. Biochemistry 2002; 41:15093 - 104; http://dx.doi.org/10.1021/bi026455d; PMID: 12475259
- Aumiller JJ, Mabashi-Asazuma H, Hillar A, Shi X, Jarvis DL. A new glycoengineered insect cell line with an inducibly-mammalianized protein N-glycosylation pathway. Glycobiology 2011; PMID: 22042767
- Palmberger D, Rendić D, Tauber P, Krammer F, Wilson IB, Grabherr R. Insect cells for antibody production: evaluation of an efficient alternative. J Biotechnol 2011; 153:160 - 6; http://dx.doi.org/10.1016/j.jbiotec.2011.02.009; PMID: 21477625
- Palmberger D, Wilson IBH, Berger I, Grabherr R, Rendic D. SweetBac: a new approach for the production of mammalianised glycoproteins in insect cells. PLoS One 2012; 7:e34226; http://dx.doi.org/10.1371/journal.pone.0034226; PMID: 22485160