Abstract
This invited commentary covers the period 1997–2012 and has seen changes in terminology that progressed from “basic” and “applied” to “translational” research. In the context of Bioengineered, these changes map readily onto the processes of identifying microbial characteristics appropriate for specific applications, isolation of suitable cultures, strain or genome manipulation and exploitation of these or their metabolomes across a range of settings.
To a great degree, this commentary and my career reflect an engagement with molecular microbiology and the trialling of bacteria and derived constructs in applications ranging from intensive-scale crop protection to amelioration of gastrointestinal disease. This engagement began with laboratory and field evaluations of biocontrol, specifically use of pseudomonads effective against nematode and fungal plant pathogens, characterization of mechanisms mediating beneficial effects of probiotic lactobacilli and bifidobacteria and assessment of functional foods in multinational clinical trials relating to inflammatory bowel disease.
Subsequent work focused on (1) intellectual property (IP)-based medical devices for localized delivery of systemically toxic and gene cancer therapies; (2) growth of the science base supporting expansion of a multinational business including company acquisitions; (3) complementing existing inter-institutional research capabilities through development of a national industry-led collaboration; and, most recently, (4) strategic research programs at Ireland’s newest medical school.
My activities as outlined above parallel two distinct aspects of translational research: (1) involvement in knowledge-driven (commercial and research) organizations that brought together necessary resources and infrastructure and (2) availability of scale research funding from European Framework and Irish national programs.
Introduction—A Tale of Two Centers: Biocontrol at BioMerit Research Centre and Centre for Microbial Ecology
I finished my PhD in 1997 having been supervised by Fergal O’Gara and working alongside Yvan Moënne-Loccoz and David Dowling, at the BioMerit Research Centre in University College Cork, Ireland. At the time I joined this group, Fergal O’Gara and colleagues had published extensively in the field (pun intended!) of plant-microbe interactionsCitation1,Citation2 and, most excitingly for me, had begun exploring the mechanisms mediating bacterial survival, adaptation and response to stimuli in their environment.Citation3,Citation4 By 1997, the team had established a brand of pragmatic molecular microbiology, deriving insight into regulation of antifungal metabolites,Citation5,Citation6 uptake of essential nutrients,Citation4,Citation7 biodegradation potential and responses to plant hosts or pathogens.Citation8-Citation11 My contribution related to biocontrol—in this case the introduction of beneficial bacteria to protect target crop plants from natural pests - specifically, protection of potatoes from attack by nematodesCitation12 and sugar beet from the fungus Pythium.Citation13,Citation14 I had isolated the bacterial strain used in most of this work and had to deal with challenges associated with it being a relatively unfamiliar species. However, since that time, Pseudomonas AKA Xanthamonas AKA Stenotrophomonas maltophilia is now considered ubiquitous,Citation15 the genome of our workhorse Pseudomonas fluorescens strain (F113) has been sequenced,Citation16 and microbial interaction, as we had begun to perceive it, has evolved as quorum sensing.Citation17
Working in Fergal O’Gara’s group was a unique experience. Infamous Friday morning meetings and journal clubs were intense and sometimes intimidating affairs where, as a callow researcher, lessons regarding careful preparation were indelibly learned. Our research was at the frontier of the applied microbiology that I loved and was relevant to the scientific debates of the time. The concept of genetically modified organisms was contentious and, in a parallel and alternative approach to field testing of genetically modified crops, we were exploring potential pesticide replacement/reduction through use of naturally occurring or modified bacteria. In hindsight, the scale of the well-resourced team was unusual, with international visitors and opportunities for foreign travel to present at scientific conferences relatively frequent. Today, a cursory search of the European Union’s CORDIS site testifies to a research group that, in the 1990s, was competing successfully for Third and Fourth Framework funding and was developing interactions and partnerships across Europe. My work benefited considerably from the experiences of preparing some of those successful grant applications and from the subsequent funding. In particular, I worked for a time in the National Science Foundation - Centre for Microbial Ecology (NSF-CME) at Michigan State University where, working with Frans de Bruijn, I modified the lux-bearing transposon subsequently used in the last and most technologically sophisticated, phase of my PhD.Citation14 This phase of my PhD and early post-doctorate work confirmed my interest in host-microbe interactions. I left the O’Gara group indebted to Fergal for the opportunities he provided me and knowing that my relatively uncommon experience of EU and commercial funding would be an advantage in a postdoctoral role.
.
Probiotics at Ireland’s National Food Biotechnology Centre
As an undergraduate, I had worked for a period at Chr Hansen Laboratories in the UK where John Lyne had introduced me to practical challenges of using lactic acid bacteria (LAB) in industrial production of cheese and yoghurt (I learned to detest plaque assays). However, by 1998, I was aware that LAB were being assessed for applications in clinical settings. Specifically, Fergus Shanahan had returned to Cork from Los Angeles and, with Gerry O’Sullivan, Kevin Collins, Gerald Fitzgerald and Charlie Daly, had established a screening program for LAB with potential health-enhancing properties isolated from resected human tissue. Emergence of probiotic research in the National Food Biotechnology Centre (NFBC) and later “pharmabiotics” at the Alimentary Pharmabiotic Centre, has already been comprehensively described.Citation18 In 1998, I was considering a move to Switzerland when the NFBC provided an opportunity to build on my foundation of host-microbe interactions while also presenting me with the new challenge of clinical trial regulations and the complexities of IP management. I have a clear memory of my first meeting with Fergus Shanahan where he described his ambitions for probiotic research and discussed in very human terms the challenges (and the potential rewards and development opportunities) associated with realizing that vision. There was no contest. I emailed the Zurich group that day to say that I, like every loyal Corkonian, was going to stay at home.
Within a short time, we had demonstrated that selected orally-administered Lactobacillus salivarius strains could become established and persist in the murine intestine and, importantly, could be enumerated when excreted.Citation19 We also completed a randomized controlled trial of lactobacilli, demonstrating effective delivery, transit and influence on microflora of healthy volunteers,Citation20 and an assessment of probiotic strains in animal models of intestinal disease.Citation21
In 2001, Tiina Mattila-Sandholm (then a Professor at VTT Biotechnology in Helsinki and has since had great success at dairy giant Valio) secured European Framework funding for “PROEUHEALTH—The Food, GI-tract Functionality and Human Health Cluster.” Gathering researchers from across Europe (64 research partners from 16 European countries in 8 projects), this initiative built on a previous EU-funded collaboration which had demonstrated nutritional functionality of probiotic foods and had proven the effects of probiotic cultures on intestinal microbiota and human health using properly controlled human clinical studies (the PROBDEMO project).Citation22 Having obtained this funding, we were then obliged to complete PROGID (Probiotics and gastrointestinal disorders—controlled trials of European Union patients) or suffer the wrath of the charismatic Finn.
In actuality, the project was a tremendously educational and enjoyable experience. I have never again worked with as effective and collegiate a grouping. From our Cork base, the Shanahan team developed ethical approval applications, clinical trial protocols, case report documentation and product distribution and sample collection logistics for clinical teams in Ireland, Finland (Kuopio), the Netherlands (Wageningen) and Spain (Barcelona). As a result, probiotics strains, fermented to stable high numbers and distributed from Cork, were shown to persist in the colon of the Finnish ulcerative colitis patients who had consumed them,Citation23 and, working with Atte Von Wright, we were able to understand the mechanisms mediating this in vivo persistence.Citation24 Subsequent work with Peter Kelly developed this considerably further, with a proteomic/MALDI-TOF analysis identifying the responsible probiotic cell wall-associated adhesins.Citation25 By the end of 2001, I was part of a well-oiled team led by Fergus Shanahan that, like some others, had developed considerable expertise in microbial ecology of the healthy and diseased intestinal tract.Citation26,Citation27 I left this group to become General Manager of a cancer research center and, in hindsight, I now recognize the early signs of just how much potential the burgeoning Alimentary Pharmabiotic Centre had.Citation28
Cancer Research: Mercy Hospital and BioSciences institute
In dealing with European Framework grants, I had seen the required administrative documentation evolve into extremely comprehensive consortia agreements that, arising from a “bang for buck” emphasis on outcomes and societal benefit, identified pre-existing knowledge and outlined models for foreground IP “exploitation.” I had also been involved as Alimentary Health Ltd (a spin-out company) partnered with multi-national industry and was familiar with the benefits and constraints associated with these relationships (non-disclosure agreements, etc.). Either because of, or due to these experiences, I was attracted when an early-stage research center dedicated solely to cancer research needed a General Manager to structure and coordinate its expansion.
The Cancer Research Centre (CRC) at the Mercy Hospital in Cork was established following successes by Gerry O’Sullivan, Kevin Collins and Fergus Shanahan in the areas of micrometastasisCitation29 and, especially, high profile papers describing the apoptosis-related “Fas Counterattack,” whereby colon cancer cells resist T cell cytotoxicity while themselves expressing an apoptotic death signal (ligand) to which activated T cells are inherently sensitive.Citation30 By the time I was appointed, the Director, Gerry O’Sullivan, had identified novel methods of therapy delivery as a theme to be expanded, including genetically-enhanced bacteria.Citation31
Gerry O’Sullivan was a force of nature. He passed away in early 2012 having been mentor to innumerable surgeons and scientists and a friend to many who had learned to dread any conversation that began with Gerry saying “I had an idea…” or “I went for a walk…” (which invariably meant that while walking an idea had occurred to him). His research activities had, in fact, included resecting the tissue from which the aforementioned Cork probiotic strains had been isolated and it was as part of related work that I had met him.
In 2001, the CRC was exclusively reliant on philanthropic generosity. The board, dominated by industry members, required more sustainable research funding. Over the next three years, we secured competitive grants from the European Framework programs, Irish Health Research Board and Cancer Society and, crucially, with guidance from Ruth Davis (now with the Irish Higher Education Authority) we were able to access capital funds from the Irish Government’s Programme for Research in Third level Institutions (PRTLI). The latter meant that the center could, to a large degree, relocate to customized laboratories in the newly constructed BioSciences Institute.
By this time, core research themes had matured. While apoptosis-related work remained important,Citation32,Citation33 industry-commissioned work (IP-driven) and European funding led to development of innovative medical devices (based on blood cell disruption by ultrasound or induction of tumor cell porosity via microneedles and/or electroporation) for gene and systemically-toxic drug delivery for cancer treatment.Citation34,Citation35 Much of this came about because of collaborations with colleagues in the Irish National Microelectronics Research Centre (now Tyndall National Institute), the UK (Les Russell and John Preston), France (Lluis Mir and Michel Marty), Denmark (Julie Gehl) and Italy (Rugerro Cadossi). By mid-2004, we had progressed innovations through laboratory and animal testing, had developed proprietary IP and had begun clinical trials in palliative patients. Promising results pointed to further growth potential but the commercial world beckoned.
Beyond Research
Glanbia is a mid-sized multinational business that, in 2004, had expanded its strategy to include bioactives. This expansion involved establishing two core innovation centers (in Ireland and the USA) in addition to localization/product development teams in Nigeria, China, Germany and the UK. Known best as a producer of consumer dairy products and as a business to business (B2B) supplier of cheese and dairy-derived ingredients, the Nutritionals businesses were tasked with enhancing revenues by diversifying from commodity products. As Director of Research for the Nutritionals EMEA businesses I had responsibility for technical due diligences associated with company acquisitions, licensing and acquisition of novel products and technologies, IP management and, in many cases, proof of concept studies (often clinical trials) and business development related to new high margin bioactives delivering anti-hypertensive, anti-hypercholesterolemia, anti-infective, body composition and human and animal performance benefits. For confidentiality reasons I am unable to describe these activities and, so, will instead concentrate on two aspects of the role that particularly interested me, (1) challenges of open innovation and (2) establishment of Food For Health Ireland.
Open innovation has been promoted as a process by which commercial groups “can and should use external ideas as well as internal ideas and internal and external paths to market, as the firms look to advance their technology”Citation36. Although collaboration, particularly between universities and companies, predates this concept, open innovation has proved a remarkably successful source of new products and technologies for many companies. Of these, Procter and Gamble’s (P&G) Connect and Develop™ (C&D) corporate innovation platform is probably the best knownCitation37 and, based on the success and growth of C&D, many companies and inventors/innovators have been attracted by the potential benefits of this new way of working. Today, C&D and open innovation are associated with short-termism and inadvertent slowing of the pace of innovation, which is evident when internal R&D capability is reduced in companies and research spending is linked to immediate rather than sustained profit (i.e., incremental development rather than breakthrough research).
As research champion in Glanbia, I began to see many examples of how open innovation could be suitable for large resource-enabled multinational companies (MNC), but is ill-suited to small and mid-size companies. To my mind, open innovation assumes incorrectly that the partners involved (innovators and industry) possess the capabilities and capacities necessary to: (2) identify and appropriately protect innovations (IP) (innovators and industry); (2) develop these to a stage where they may be marketed (innovators and industry); (3) negotiate with venture capitalists or other investors (innovators and industry); (4) communicate the characteristics/benefits of the innovations to potential buyers (innovators and industry); (5) understand what is being offered by innovators (industry); (6) recognize the regulatory requirements, market potential and level of investment required to pilot and scale-up manufacture (innovators and industry); (7) understand that innovations may occasionally be sourced from larger MNCs when the scale of the opportunity or market position, despite investment and development, no longer fits implementation of their strategy and they wish to divest (industry).
Simply put, it was clear to me and counterparts in competitor companies and Irish Government agencies that, for “Ireland Inc.” to exploit the considerable research infrastructure of Irish research institutes and universities, collaborative funding and sharing of expertise would be required.
Following extremely lengthy dialog between the companies involved (Glanbia, Dairygold, Kerry, Carbery) mediated by Enterprise Ireland (Paul Roben and John Mulvihill) and review of work-plans submitted by academic researchers, Food for Health Ireland (www.fhi.ie) was established in 2009. As this was an industry-led research initiative to accelerate mining and testing of milk-derived bioactives for eventual commercial use, considerable emphasis was placed on avoiding challenges associated with State Aid rules, provision of opportunity for additional industry involvement and appropriate management for commercializing any foreground IP that might result from the funding. Direct plus overhead contributions to the research institutes, combined with industry investment, provided more than €20 million in research support and, in 2009, represented the largest investment made by Enterprise Ireland in an R&D consortium since its launch in 1998. At the time of writing, this initiative continues and second round funding is anticipated.
.
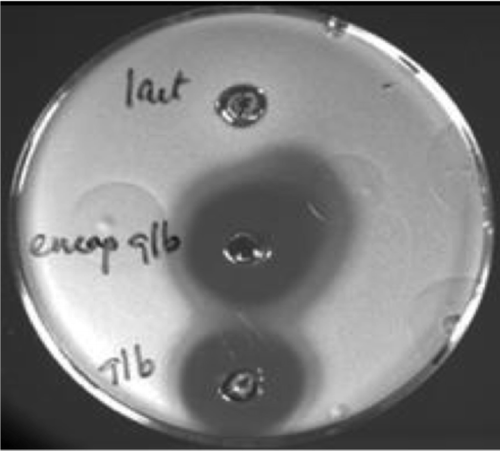
Figure 2. A plate asay demonstrating inhibition of C. difficile (clinical isolate) growth by an (1) encapsulated and (2) non-protected naturally occurring fatty acid. The comparative control is a bacteriocin. Encapsulation enables consumption and maintenance of efficacy in the intestines while, in the unprotected form, the FA demonstrates emolient properties considered an advantage over harsh alcohol-based hand hygiene alternatives. Previously published in: Flanagan J, Su J, O’Brien C, O’Riordan B, Singh H, Dunne, C. Microencapsulating properties of Acacia (sen) SUPER GUMTM . Foods and Food Ingredients Journal of Japan 2008; 213: 256–62.
Conclusions
In late 2009 I was appointed Chair of Research at the Graduate Entry Medical School at the University of Limerick, Ireland. We have, since then, recruited professorial researchers across the principal medical and surgical specialties and have established the Centre for Interventions in Infection, Inflammation and Immunity—known as 4i (www.4i.ie)—to provide the type of supports detailed above to colleagues both on campus and across our affiliated clinical sites. My research interests continue to reflect my personal, scientific and business experiences.
Acknowledgments
I am hugely appreciative of friends and colleagues from the BioMerit Research Centre (UCC), Centre for Microbial Ecology/Plant Research laboratories (MSU), Irish National Food Biotechnology Centre, Cork Cancer Research Centre, Glanbia Innovation Centres and the University of Limerick’s Graduate Entry Medical School where I currently ply my trade. Special thanks are due to Fergal O’Gara, Fergus Shanahan and the late Gerry O’Sullivan (for their friendship and advice), to the Irish Health Research Board, Enterprise Ireland, National Children’s Research Centre, European Commission Framework Programs and the many commercial partners who have provided research funding. I am forever grateful to Suzanne, Pat, Eileen, Kevin, Louise, Collette and Bernard.
References
- Birkenhead K, Manian SS, O’Gara F. Dicarboxylic acid transport in Bradyrhizobium japonicum: use of Rhizobium meliloti dct gene(s) to enhance nitrogen fixation. J Bacteriol 1988; 170:184 - 9; PMID: 3422072
- Wang YP, Birkenhead K, Boesten B, Manian S, O’Gara F. Genetic analysis and regulation of the Rhizobium meliloti genes controlling C4-dicarboxylic acid transport. Gene 1989; 85:135 - 44; http://dx.doi.org/10.1016/0378-1119(89)90473-3; PMID: 2695394
- Stephens PM, O’sullivan M, O’gara F. Effect of Bacteriophage on Colonization of Sugarbeet Roots by Fluorescent Pseudomonas spp. Appl Environ Microbiol 1987; 53:1164 - 7; PMID: 16347343
- O’Sullivan DJ, O’Gara F. Regulation of iron assimilation: nucleotide sequence analysis of an iron-regulated promoter from a fluorescent pseudomonad. Mol Gen Genet 1991; 228:1 - 8; PMID: 1679522
- Fenton AM, Stephens PM, Crowley J, O’Callaghan M, O’Gara F. Exploitation of gene(s) involved in 2,4-diacetylphloroglucinol biosynthesis to confer a new biocontrol capability to a Pseudomonas strain. Appl Environ Microbiol 1992; 58:3873 - 8; PMID: 1476431
- Carroll H, Moenne-Loccoz Y, Dowling DN, O’gara F. Mutational Disruption of the Biosynthesis Genes Coding for the Antifungal Metabolite 2,4-Diacetylphloroglucinol Does Not Influence the Ecological Fitness of Pseudomonas fluorescens F113 in the Rhizosphere of Sugarbeets. Appl Environ Microbiol 1995; 61:3002 - 7; PMID: 16535101
- Sexton R, Gill PR Jr., Callanan MJ, O’Sullivan DJ, Dowling DN, O’Gara F. Iron-responsive gene expression in Pseudomonas fluorescens M114: cloning and characterization of a transcription-activating factor, PbrA. Mol Microbiol 1995; 15:297 - 306; http://dx.doi.org/10.1111/j.1365-2958.1995.tb02244.x; PMID: 7746151
- O’Sullivan DJ, O’Gara F. Delivery system for creation of one-step in vivo lac gene fusions in Pseudomonas spp. involved in biological control. Appl Environ Microbiol 1988; 54:2877 - 80; PMID: 2850764
- Ramos JL, Díaz E, Dowling D, de Lorenzo V, Molin S, O’Gara F, et al. The behavior of bacteria designed for biodegradation. Biotechnology (N Y) 1994; 12:1349 - 56; http://dx.doi.org/10.1038/nbt1294-1349; PMID: 7765565
- Fedi S, Brazil D, Dowling DN, O’Gara F. Construction of a modified mini-Tn5 lacZY non-antibiotic marker cassette: ecological evaluation of a lacZY marked Pseudomonas strain in the sugarbeet rhizosphere. FEMS Microbiol Lett 1996; 135:251 - 7; http://dx.doi.org/10.1111/j.1574-6968.1996.tb07997.x; PMID: 8595865
- Fedi S, Tola E, Moënne-Loccoz Y, Dowling DN, Smith LM, O’Gara F. Evidence for signaling between the phytopathogenic fungus Pythium ultimum and Pseudomonas fluorescens F113: P. ultimum represses the expression of genes in P. fluorescens F113, resulting in altered ecological fitness. Appl Environ Microbiol 1997; 63:4261 - 6; PMID: 9361412
- Cronin D, Moenne-Loccoz Y, Fenton A, Dunne C, Dowling DN, O’gara F. Role of 2,4-Diacetylphloroglucinol in the Interactions of the Biocontrol Pseudomonad Strain F113 with the Potato Cyst Nematode Globodera rostochiensis. Appl Environ Microbiol 1997; 63:1357 - 61; PMID: 16535571
- Dunne C, Crowley JJ, Moënne-Loccoz Y, Dowling DN, de Bruijn FJ, O’Gara F. Biological control of Pythium ultimum by Stenotrophomonas maltophilia W81 is mediated by an extracellular proteolytic activity. Microbiol 1997; 143:3921 - 31; http://dx.doi.org/10.1099/00221287-143-12-3921
- Dunne C, Moënne-Loccoz Y, de Bruijn FJ, O’Gara F. Overproduction of an inducible extracellular serine protease improves biological control of Pythium ultimum by Stenotrophomonas maltophilia strain W81. Microbiology 2000; 146:2069 - 78; PMID: 10931911
- Hayward AC, Fegan N, Fegan M, Stirling GR. Stenotrophomonas and Lysobacter: ubiquitous plant-associated gamma-proteobacteria of developing significance in applied microbiology. J Appl Microbiol 2010; 108:756 - 70; http://dx.doi.org/10.1111/j.1365-2672.2009.04471.x; PMID: 19702860
- Redondo-Nieto M, Barret M, Morrisey JP, Germaine K, Martínez-Granero F, Barahona E, et al. Genome sequence of the biocontrol strain Pseudomonas fluorescens F113. J Bacteriol 2012; 194:1273 - 4; http://dx.doi.org/10.1128/JB.06601-11; PMID: 22328765
- Baysse C, Cullinane M, Dénervaud V, Burrowes E, Dow JM, Morrissey JP, et al. Modulation of quorum sensing in Pseudomonas aeruginosa through alteration of membrane properties. Microbiology 2005; 151:2529 - 42; http://dx.doi.org/10.1099/mic.0.28185-0; PMID: 16079332
- Hill C. Probiotics and pharmabiotics: alternative medicine or an evidence-based alternative?. Bioeng Bugs 2010; 1:79 - 84; http://dx.doi.org/10.4161/bbug.1.2.10796; PMID: 21326932
- Murphy L, Dunne C, Kiely B, Shanahan F, O’Sullivan GC, Collins JK. In vivo assessment of potential probiotic Lactobacillus salivarius strains: evaluation of their establishment, persistence and localisation in the murine gastrointestinal tract. Microb Ecol Health Dis 1999; 11:149 - 57; http://dx.doi.org/10.1080/089106099435727
- Collins JK, Dunne C, Murphy L, Morrissey D, O’Mahony L, O’Sullivan E, et al. A randomised controlled trial of a probiotic Lactobacillus strain in healthy adults: Assessment of its delivery, transit and influence on microbial flora and enteric immunity. Microb Ecol Health Dis 2002; 14:81 - 9; http://dx.doi.org/10.1080/08910600260081720
- Dunne C, Murphy L, Flynn S, O’Mahony L, O’Halloran S, Feeney M, et al. Probiotics: from myth to reality. Demonstration of functionality in animal models of disease and in human clinical trials. Antonie Van Leeuwenhoek 1999; 76:279 - 92; http://dx.doi.org/10.1023/A:1002065931997; PMID: 10532384
- Mattila-Sandholm T. The PROBDEMO project: demonstration of the nutritional functionality of probiotic foods. Trends Food Sci Technol 1999; 10:385 - 6; http://dx.doi.org/10.1016/S0924-2244(00)00026-1
- Von Wright A, Vilpponen-Salmela T, Pagès Llopis M, Collins K, Kiely B, Shanahan F, et al. The survival and colonic adhesion of Bifidobacterium longum infantis in patients with ulcerative colitis. Int Dairy J 2002; 12:197 - 200; http://dx.doi.org/10.1016/S0958-6946(01)00162-5
- Dunne C, Kelly P, O’Halloran S, Soden D, Bennett M, Von Wright A, et al. Mechanisms of adherence of a probiotic Lactobacillus strain during and post-in vivo assessment in ulcerative colitis patients. Microb Ecol Health Dis 2004; 16:96 - 104; http://dx.doi.org/10.1080/08910600410032295
- Kelly P, Maguire PB, Bennett M, Fitzgerald DJ, Edwards RJ, Thiede B, et al. Correlation using proteomic analysis of probiotic Lactobacillus salivarius growth phase with presence of a cell wall-associated adhesin. FEMS Microbiol Lett 2005; 252:153 - 9; http://dx.doi.org/10.1016/j.femsle.2005.08.051; PMID: 16214296
- Dunne C. Adaptation of bacteria to the intestinal niche: probiotics and gut disorder. Inflamm Bowel Dis 2001; 7:136 - 45; http://dx.doi.org/10.1097/00054725-200105000-00010; PMID: 11383587
- Duffy M, O’Mahony L, Coffey JC, Collins JK, Shanahan F, Redmond HP, et al. Sulfate-reducing bacteria colonize pouches formed for ulcerative colitis but not for familial adenomatous polyposis. Dis Colon Rectum 2002; 45:384 - 8; http://dx.doi.org/10.1007/s10350-004-6187-z; PMID: 12068199
- Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012; 488:178 - 84; http://dx.doi.org/10.1038/nature11319; PMID: 22797518
- O’Sullivan GC, Collins JK, Kelly J, Morgan J, Madden M, Shanahan F. Micrometastases: marker of metastatic potential or evidence of residual disease?. Gut 1997; 40:512 - 5; PMID: 9176080
- O’Connell J, Houston A, Bennett MW, O’Sullivan GC, Shanahan F. Immune privilege or inflammation? Insights into the Fas ligand enigma. Nat Med 2001; 7:271 - 4; http://dx.doi.org/10.1038/85395; PMID: 11231613
- O’Sullivan GC, Aarons SJ, Shanahan F. Mutant salmonella as vectors for gene therapy. Gastroenterology 2001; 121:224 - 6; http://dx.doi.org/10.1016/S0016-5085(01)90011-3; PMID: 11438515
- Ryan P, McCarthy S, Kelly J, Collins JK, Dunne C, Grogan L, et al. Prevalence of bone marrow micrometastases in esophagogastric cancer patients with and without neoadjuvant chemoradiotherapy. J Surg Res 2004; 117:121 - 6; http://dx.doi.org/10.1016/j.jss.2003.12.008; PMID: 15013722
- Ambrose M, Ryan A, O’Sullivan GC, Dunne C, Barry OP. Induction of apoptosis in renal cell carcinoma by reactive oxygen species: involvement of extracellular signal-regulated kinase 1/2, p38delta/gamma, cyclooxygenase-2 down-regulation and translocation of apoptosis-inducing factor. Mol Pharmacol 2006; 69:1879 - 90; http://dx.doi.org/10.1124/mol.105.020875; PMID: 16543392
- Larkin J, Soden D, Collins C, Tangney M, Preston JM, Russell LJ, et al. Combined electric field and ultrasound therapy as a novel anti-tumour treatment. Eur J Cancer 2005; 41:1339 - 48; http://dx.doi.org/10.1016/j.ejca.2005.01.025; PMID: 15913991
- Soden DM, Larkin JO, Collins CG, Tangney M, Aarons S, Piggott J, et al. Successful application of targeted electrochemotherapy using novel flexible electrodes and low dose bleomycin to solid tumours. Cancer Lett 2006; 232:300 - 10; http://dx.doi.org/10.1016/j.canlet.2005.03.057; PMID: 15964138
- Chesbrough HW. Open Innovation: The new imperative for creating and profiting from technology. Boston: Harvard Business School Press; 2003.
- Huston L, Sakkab N. Connect and Develop Inside Procter & Gamble’s New Model for Innovation. Harv Bus Rev 2006; 84:58 - 66
