Abstract
Most members of the genus Bifidobacterium are commensals of the human gastrointestinal tract and some strains were shown to exert beneficial effects on their host. Based on these effects and due to their status as GRAS (generally recognized as safe) microorganisms, specific strains of bifidobacteria are marketed as probiotics. Despite their important role in food and dairy industries, the mechanisms responsible for the probiotic effects of bifidobacteria are mostly unknown. Over the last decade, the genomes of a large number of bifidobacteria have been sequenced and analyzed. This has yielded a number of genes and their products that are speculated to contribute to the probiotic effects of bifidobacteria. The gold standard to demonstrate a role for specific genes is the analysis of mutants. At present, only a small number of mutants of bifidobacteria have been generated by targeted mutagenesis. This is owed to the genetic inaccessibility of most strains and a lack of appropriate molecular tools. Successful generation of mutants of bifidobacteria was achieved by various methods including classical suicide vector strategies, increase of transformation efficiencies by methylation of plasmids and the use of temperature-sensitive vectors. In this commentary, we will describe the methods successfully used for mutagenesis of bifidobacteria and discuss their advantages and limitations.
Introduction
Bifidobacteria are Gram-positive microorganisms with a high-GC content that belong to the Actinobacteria phylum.Citation1 Most bifidobacteria are commensals of the intestinal tract of humans and animalsCitation2 and some strains were shown to have beneficial effects on the health status of their hosts. The health-promoting effects described include the production of vitamins, prevention of diarrhea, reduction of cholesterol levels, treatment of irritable bowel syndrome and inflammatory bowel disease, immunostimulation and cancer prevention (reviewed in refs. Citation1,Citation3 and Citation4). Due to these effects, bifidobacteria have attracted considerable commercial interest and are used in a large number of probiotic formulations. Despite their economic importance, the mechanisms that are responsible for the probiotic effects of bifidobacteria are far from understood. The genomes of a number of strains of different species have been sequenced and annotated and are publically available.Citation1,Citation5 However, the detailed analysis of the probiotic effects of bifidobacteria is hampered by the lack of appropriate tools for their genetic modification. While there has been some progress in the development of expression vectors, the currently available protocols do not yield transformation efficiencies above the threshold required to achieve chromosomal integration of non-replicative vectors by homologous recombination.Citation5
The gold standard to investigate the role of single genes and their products is site-directed mutagenesis and the subsequent phenotypic analysis of the obtained mutants. So far, no system for bifidobacteria has been described that allows for directed mutagenesis in at least a number of different strains and species. As a consequence, generation of mutants by site-directed integration of deletion constructs has been described only for a very limited number of genes in a handful of strains and only a single Bifidobacterium breve strain has been mutated repeatedly.
Suicide Vectors for Mutagenesis in Bifidobacteria
In B. longum NCC2705, the bl0033 gene encoding the substrate-binding protein of a fructose-specific ABC-type sugar transporterCitation6 has been disrupted by a classical approach using a suicide vector. The ABC transporter was shown to confer resistance to infection with Escherichia coli O157:H7 in a murine model via increased acetate production.Citation7 For the targeted disruption of bl0033, two 1-kb fragments flanking the gene were cloned up- and downstream of a spectinomycin resistance cassette into pBluescriptIISK(+), an E. coli cloning vector that is non-replicative in bifidobacteria. After electroporation into B. longum NCC2705, transformants were selected with spectinomycin and disruption of bl0033 was confirmed by PCR.Citation7 However, the transformation protocol used was described to yield a maximum of 1.6 × 104 colony-forming units (cfu) per µg DNA in B. longum strains,Citation8 which is below what is required to allow for homologous recombination. In fact, in a later publication the authors admitted that the process was indeed very time consuming since it took more than one year to obtain a single clone of the mutant.Citation9 Accordingly, there are no further reports on mutants generated with this system in B. longum NCC2705 or other bifidobacteria.
Plasmid Artificial Modification to Increase Transformation Efficiencies
The number of available genome sequences of different strains of various Bifidobacterium species has been increasing over the last decade. In all of the 23 fully sequenced and annotated bifidobacterial genomesCitation5 restriction-modification (R-M) systems have been identified.Citation10 R-M systems of bacteria have evolved to limit the uptake of foreign DNA, e.g., upon infection with a bacteriophage and typically consist of a DNA methyltransferase (MTase) and a restriction endonuclease (REase).Citation11 Both enzymes recognize the same DNA motifs. However, while the MTase is responsible for the methylation of these motifs, the REase cleaves any DNA that is not methylated in the pattern specific for the host.Citation12 It is thus not surprising that R-M systems are one of the problems for genetic modification and the more R-M systems a bacterium encodes the more recalcitrant to manipulation it usually is. An approach, which is increasingly used to improve the genetic accessibility of bacteria that are difficult to manipulate, is the methylation of vectors in a pattern specific for the target organisms. This is achieved by using either E. coli cloning hosts expressing the respective MTases or by in vitro methylation using recombinant purified MTases.Citation13-Citation16
Several bifidobacteria were shown to possess more than one R-M system and the methyltransferases of the respective strains were successfully expressed in E. coli cloning hosts.Citation17,Citation18 Using this method termed plasmid artificial modification (PAM), the transformation efficiency of B. adolescentis ATCC15703 was increased from 1–3 × 100 cfu/µg DNA for unmethylated DNA to up to 4 × 105 cfu/µg when plasmid was isolated from an E. coli TOP10 strain harbouring the two MTases of B. adolescentis ATCC15703.Citation18 Similarly, transformation efficiencies of B. breve UCC2003, which harbours three R-M systems, was improved from about 1 × 104 cfu/µg to about 1 × 107 cfu/µg with pAM5 isolated from E. coli EC101pNZ-MBbrII-MBbrIII, a strain expressing two of the three MTase genes.Citation17 The high transformation efficiencies obtained with plasmids isolated from this E. coli cloning host allowed for the successful insertional mutagenesis of apuB and galE in B. breve UCC2003 using non-replicative plasmids. Both mutants were confirmed by Southern blot and phenotypic characterization.Citation17 Since then, a number of other genes were successfully inactivated in B. breve UCC2003 using PAM including cldE, a component of a cellodextrin ABC transporter,Citation19 the tadA gene encoding the ATPase of tight adherence pili,Citation20 Bbr_0430 encoding the priming glycosylase involved in the synthesis of extracellular polysaccharideCitation21 and several genes of two-component systems.Citation22,Citation23
PAM has proven successful to increase transformation efficiencies of B. breve UCC2003 above levels required for site-directed recombination with non-replicative vectors. We thus sought to apply this method to generate mutants of B. bifidum S17. This strain is a promising probiotic candidate, which adheres tightly to various intestinal epithelial cell lines in a process dependent on BopA, a lipoprotein of the cell envelope.Citation24-Citation26 B. bifidum S17 exhibits potent anti-inflammatory effects by inhibition of LPS-induced NF-κB activation and pro-inflammatory cytokine secretion in cultured intestinal epithelial cells and protects from intestinal inflammation in different models of colitis.Citation25,Citation27,Citation28 Analysis of the recently sequenced and annotated genome sequenceCitation29 revealed two putative R-M systems (). Additional MTase genes were identified for which no corresponding REase genes were found (data not shown). Homology to other MTase genes indicates that these genes encode RNA-specific methyltransferases and they were thus excluded from further analysis.
Figure 1. (A) Genetic organization and expression of the genes of the Type I R-M system of B. bifidum S17. The hsdM, hsdS and hsdR genes (black arrows) encoding the methyltransferase, sequence recognition and restriction subunit are located in close proximity to a putative integrase gene (gray). (B) Genetic organization and expression of the genes encoding the Type II R-M system of B. bifidum S17 with methyltransferase (bbif0710) and restriction endonuclease genes (bbif0711; black arrows) and the adjacent integrase gene (gray). Expression of all genes was analyzed in RNA samples of bacteria harvested in exponential growth phase by reverse transcription PCR. Negative controls (no reverse transcription; middle) and positive controls (PCR on chromosomal DNA, right bands) were included. Gels were loaded with samples as follows: RT-PCRs in lanes 3, 6 and 9 (3: hsdM; 6: hsdS and 9: hsdR in A; 3: bbif0711 and 6: bbif0711 in B) and corresponding negative (lanes 1, 4, 7) and positive controls (lanes 2, 5 and 8).
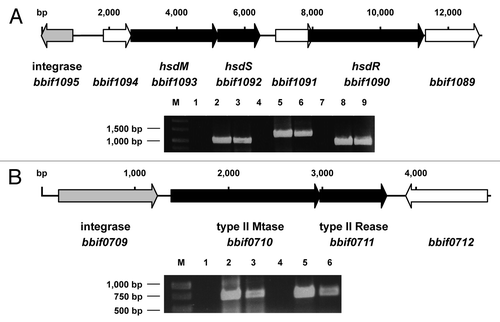
One of the R-M systems shows the typical features of a Type I system with hsdM encoding for the methyltransferase subunit, hsdS for the subunit recognizing the specific sequence motif and hsdR for the endonuclease subunit (). The genes of the Type I R-M system are inserted into a cluster of genes encoding for enzymes of the arginine biosynthesis pathway (data not shown) and show a markedly lower GC-content (53%) compared to the rest of the chromosome (62.8%). Downstream, a putative integrase gene (bbif1095) is found indicating that the Type I R-M system was acquired by horizontal gene transfer. The deduced amino acid sequences of HsdM and HsdR show high homology to the respective subunits in other B. bifidum strains (up to 99%) and B. longum strains (over 90%). The homology for the deduced HsdS sequence is less pronounced (70% to B. bifidum PRL2010 and 52% to B. longum DJO10A) suggesting that the sequence-specificity might be different in these strains.
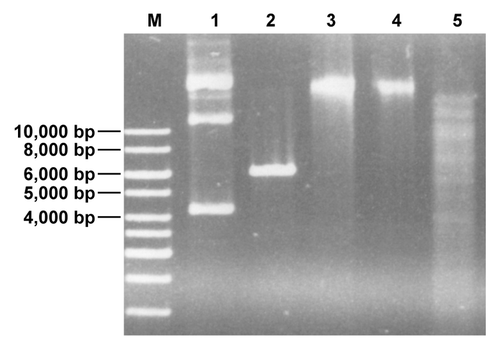
The second R-M system is a putative Type II R-M system with genes encoding for an MTase and a corresponding REase (). Immediately upstream of the Type II MTase gene another putative integrase gene (bbif0709) is located and bbif0709, the MTase and REase genes show a GC-content of 53% again indicating acquisition of the Type II R-M system by horizontal gene transfer. BLAST comparison of the deduced amino acid sequence of the putative Type II REase (BBIF7011), using the REBASE database for R-M enzymesCitation10 suggested that the enzyme might be an XhoI isoschizomer. This hypothesis was tested by performing an REase protection assay. In line with this hypothesis chromosomal DNA of B. bifidum S17 was protected from digestion with a commercial XhoI enzyme ().
Figure 2. Chromosomal DNA of B. bifidum S17 is protected from restriction with XhoI (lane 4). As controls undigested chromosomal DNA (lane 3) and EcoRI-restricted DNA (lane 5) were loaded into the neighboring slots of the gel. Additionally, untreated (lane 1) or XhoI-digested (lane 2) pIMK2 (6,190 bp), which harbors a single XhoI-site was run on the same gel.
Expression of all genes of the two putative R-M systems of B. bifidum S17 was tested by reverse-transcription PCR on RNA samples isolated from bacteria in exponential growth phase. All genes are expressed under these conditions () suggesting that both R-M systems are active in B. bifidum S17. In order to establish a PAM system to increase transformation efficiencies of B. bifidum S17 two plasmids were generated for arabinose-inducible expression of the MTases of the two R-M systems in E. coli. The Type II MTase gene and hsdM and hsdS were amplified from chromosomal DNA using primer pairs bbif0710_fwd/bbif0710_rev or hsdMS_fwd/hsdMS_rev (Table S1). The pBAD vector, a derivative of pBluescript harbouring araC encoding the arabinose repressor and the arabinose-inducible araB promoter (ParaB) of pREDI,Citation30 was created and the PCR products were cloned into this vector under control of ParaB. From these intermediate constructs, ParaB and the methyltransferase gene were amplified together with araC using the primers pBAD_fwd and pBAD_rev (Table S1). Both PCR products were cloned separately into p16S, a derivative of p16SluxCitation31 lacking the lux operon thereby creating vectors p16S_hsdMS and p16S_bbif0710. Due to their temperature sensitive replicon these vectors can be integrated into a 16S rRNA gene of a wide range of Enterobacteriaceae.Citation31 Both plamsids were transformed into E. coli ET12567, a strain that lacks own modifying enzymes.Citation32 Chromosomal integration of the vectors was induced in positive clones and integration was verified by PCR as described.Citation31
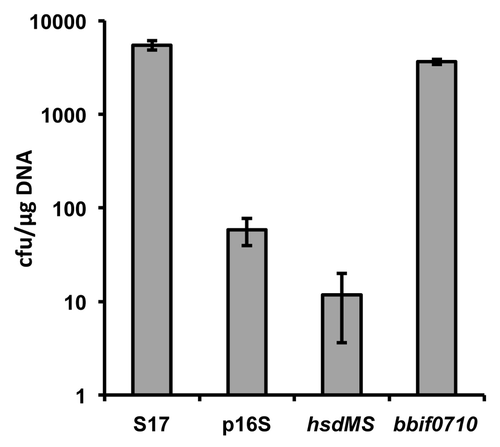
In order to test the effect of PAM on transformation efficiencies of B. bifidum S17 the E. coli/Bifidobacterium shuttle vector pMDY23Citation33 was transformed into the E. coli strains harbouring a copy of either of the two plasmids integrated into the chromosome or the control strain, i.e., E. coli ET12567 with a chromosomal copy of p16S. The pMDY23 plasmid was then re-isolated from these strains and transformed into B. bifidum S17 rendered electrocompetent using a previously described protocol.Citation34 Plasmid isolated from B. bifidum S17 served as positive control. PAM of pMDY23 by expression of hsdMS did not improve transformation efficiency (). By contrast, methylation of pMDY23 by expression of the Type II MTase in E. coli ET12567 increased transformation efficiencies of B. bifidum S17 by approx. two orders of magnitude compared with the non-methylated vector reaching approx. four × 103 cfu/µg DNA. However, transformation efficiencies did not exceed levels observed with pMDY23 isolated from B. bifidum S17. This is in line with observations made by other groups using PAM in bifidobacteriaCitation17,Citation18 and suggests that the low transformation efficiency of B. bifidum S17 (and possibly other strains) is only partially attributable to R-M systems.
Figure 3. Transformation efficiencies of B. bifidum S17 with pMDY23 isolated either from B. bifidum S17 (S17) or E. coli ET12567 harbouring a chromosomal copy of p16S (empty vector; p16S), p16S_hsdMS (vector for expression of the Type I methylase; hsdMS) or p16S_bbif0710 (vector for expression of the Type II MTase; bbif0710). Transformation efficiencies are expressed as cfu/µg plasmid DNA. Values are the mean transformation efficiencies of two independent cultures of competent cells prepared on the same day and similar results were obtained with at least further four cultures.
Use of Temperature-Sensitive Plasmids for Mutagenesis
The first report on an insertion mutant of a bifidobacterial strain was the disruption of apuB in B. breve UCC2003 encoding an extracellular type II amylopullulanase.Citation35 Gene disruption was achieved using an approach initially described for Lactococcus lactisCitation36 but also successfully applied to other Gram-positive organisms including Listeria monocytogens.Citation37 B. breve UCC2003 was first transformed with pTGB019, a derivative of the temperature-sensitive lactococcal plasmid pVE6007 harbouring a functional repA gene. A 1 kb internal fragment of apuB was then cloned into pORI19, which is non-replicative due to the lack of repA and the resulting vector pORI19-apuB was introduced into B. breve UCC2003 pTGB019. After successful introduction of both plasmids, growth temperature was shifted to 42°C, which blocks replication of pTGB019 and, as a consequence of the lack of a functional repA also of pORI19-derivatives. Presence of the antibiotic selects for integration of pORI-derivatives into the genome of B. breve UCC2003 at the desired site, in this case the apuB gene and causes gene disruption. Successful disruption of the apuB gene was shown by the inability of the mutant to grow on modified Rogosa medium containing starch, amylopectin, glycogen or pullulan as sole carbon source.Citation35 To date, this method has not been applied for the generation of other mutants in B. breve UCC2003 nor has it been used independently by other groups and in other bifidobacteria. In fact, we have tried to transform pVE6007 into B. bifidum S17 several times and could not obtain any positive clones (data not shown).
Recently, the successful generation of a temperature-sensitive plasmid for deletion of genes in B. longum 105-ACitation9 was reported. The authors amplified the repB gene of pKKT427 by error-prone PCR, replaced the repB in pKKT427 with the PCR product thereby creating a library of clones containing different repB mutants. This library was transformed into B. longum 105-A and about 3000 clones were screened for growth at 30°C and 42°C. This led to the identification of a single clone containing a temperature-sensitive plasmid. This plasmid was termed pKO403 and subsequently used to create a deletion mutant in the pyrE gene of B. longum 105-A via two homologous recombination events.Citation38 Deletion of pyrE, which encodes orotatephosphoribosyltransferase and is crucial for pyrimidine metabolism, was confirmed by resistance to 5-fluoroorotic acid and auxotrophy for uracil of the mutant. The authors further validated their system by re-creating a deletion mutant in the bl0033 gene of B. longum NCC2705,Citation9 which was obtained earlier by the same group using a classical yet very time-consuming suicide vector strategy.Citation7
Concluding Remarks
The first report on a mutant in a Bifidobacterium strain was published only 5 y ago.Citation35 Since then several groups have proposed different strategies for site-directed mutagenesis in different strains of bifidobacteria. Nevertheless, most strains remain resistant to mutagenesis mainly due to notoriously low transformation efficiencies. One of the reasons for low transformation efficiencies is that most bifidobacteria possess multiple R-M systems. Further factors leading to low transformation efficiencies have to our knowledge not been investigated systematically. However, one explanation may be differences in cell wall components between bifidobacteria and other Gram-positive organisms. For example, the teichoic acids (TA) of bifidobacteria were shown to have an unusual structure compared with the TA of other Gram-positive organisms.Citation39-Citation41 Moreover, from our own experience efficient lysis of bifidobacteria, e.g., for the preparation of crude extracts, requires lysozyme and mutanolysin. Both enzymes are murein hydrolases cleaving the β-1,4 glycosidic bond of the N-acetylmuramyl-N-acetylglucosamine backbone of peptidoglycan. However, mutanolysin was shown to have a broader spectrum of activity also cleaving peptidoglycans of group A streptococci, which are resistant to lysozyme treatment.Citation42,Citation43 This indicates that the peptidoglycan of bifidobacteria might have an altered structure resulting in a reduced sensitivity toward the protocols used to prepare competent cells.
By far the most frequently used approach to increase transformation efficiencies is PAM. Using this method a number of mutants have been generated in a B. breve strain.Citation19-Citation23 Improved transformation efficiencies using PAM were independently confirmed for a B. adolescentis strainCitation18 and by our own results (). However, in none of these cases PAM was able to increase efficiencies of transformation markedly above those observed with plasmid DNA isolated from the target organism. Thus, while PAM is undoubtedly a valuable method to overcome the R-M barrier in bifidobacteria, levels required for site-directed recombination are only obtained with strains that already show reasonable levels of intrinsic competence or are highly susceptible to the currently available protocols for the generation of competent cells such as B. breve UCC2003. Another drawback of PAM is that it is highly specific for a single strain and the respective E. coli cloning host cannot be used for other strains.
The generation of an insertion mutant in the bl0033 gene in B. longum NCC2705 using a suicide vectorCitation7 indicates that this strategy is in principal applicable for mutagenesis in bifidobacteria. In consequence, the development of new protocols for the generation of competent cells together with PAM suicide vectors might have potential for targeted mutagenesis for bifidobacteria.
The use of temperature-sensitive replicons for mutagenesis has been successfully used in a wide range of organisms. In particular, the systems based on the temperature-sensitive replicon of pVE6007 and the repA-deficient pORI plasmids was successfully used in a number of Gram-positive bacteria. To date, only one mutant of a B. breve strain has been generated using this system, possibly due to incompatibility of the replicons with the majority of bifidobacteria. More recently, pKO403, a temperature-sensitive derivative of the E. coli/Bifidobacterium shuttle vector pKKT427, was successfully applied for the generation of two mutants in B. longum NCC27755.Citation9 The pKKT427 vector is based on the pTB6 replicon, which was shown to stably replicate in strains of B. longum, B. breve and B. animalis (reviewed in ref. Citation5). Thus, at present pKO403 is the most promising approach for targeted mutagenesis in a wider range of bifidobacteria and might prove more applicable than to improve transformation efficiencies by optimizing protocols and/or PAM. Nevertheless, successful application of pKO403 for targeted mutagenesis in other strains and species of bifidobacteria needs to be confirmed.
In conclusion, despite their prominent contribution to the intestinal microbiota, the effects on human health and the large economic interest in probiotics, tools for the genetic modification of bifidobacteria are still largely missing. These tools are a fundamental basis for the analysis of the effects of bifidobacteria on the human host and the underlying mechanisms. While methods for targeted mutagenesis are in place for a very limited set of individual strains, no universal system for bifidobacteria is available and might actually prove impossible to achieve. Nevertheless, the development of temperature-sensitive plasmids for a wider range of bifidobacteria is a promising approach and worthwhile to investigate in more detail.
Additional material
Download Zip (113.8 KB)Disclosure of Potential Conflicts of Interest
No potential conflicts of interest have been disclosed.
References
- Lee J-H, O’Sullivan DJ. Genomic insights into bifidobacteria. Microbiol Mol Biol Rev 2010; 74:378 - 416; http://dx.doi.org/10.1128/MMBR.00004-10; PMID: 20805404
- Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature 2012; 486:222 - 7; PMID: 22699611
- Leahy SC, Higgins DG, Fitzgerald GF, van Sinderen D. Getting better with bifidobacteria. J Appl Microbiol 2005; 98:1303 - 15; http://dx.doi.org/10.1111/j.1365-2672.2005.02600.x; PMID: 15916644
- Picard C, Fioramonti J, Francois A, Robinson T, Neant F, Matuchansky C. Review article: bifidobacteria as probiotic agents -- physiological effects and clinical benefits. Aliment Pharmacol Ther 2005; 22:495 - 512; http://dx.doi.org/10.1111/j.1365-2036.2005.02615.x; PMID: 16167966
- Sun Z, Baur A, Zhurina D, Yuan J, Riedel CU. Accessing the inaccessible: molecular tools for bifidobacteria. Appl Environ Microbiol 2012; 78:5035 - 42; http://dx.doi.org/10.1128/AEM.00551-12; PMID: 22582076
- Wei X, Guo Y, Shao C, Sun Z, Zhurina D, Liu D, et al. Fructose uptake in Bifidobacterium longum NCC2705 is mediated by an ATP-binding cassette transporter. J Biol Chem 2012; 287:357 - 67; http://dx.doi.org/10.1074/jbc.M111.266213; PMID: 22102285
- Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011; 469:543 - 7; http://dx.doi.org/10.1038/nature09646; PMID: 21270894
- Nakamura T, Sasaki T, Fujimori M, Yazawa K, Kano Y, Amano J, et al. Cloned cytosine deaminase gene expression of Bifidobacterium longum and application to enzyme/pro-drug therapy of hypoxic solid tumors. Biosci Biotechnol Biochem 2002; 66:2362 - 6; http://dx.doi.org/10.1271/bbb.66.2362; PMID: 12506973
- Sakaguchi K, He J, Tani S, Kano Y, Suzuki T. A targeted gene knockout method using a newly constructed temperature-sensitive plasmid mediated homologous recombination in Bifidobacterium longum. Appl Microbiol Biotechnol 2012; 95:499 - 509; http://dx.doi.org/10.1007/s00253-012-4090-4; PMID: 22639142
- Roberts RJ, Vincze T, Posfai J, Macelis D. REBASE--a database for DNA restriction and modification: enzymes, genes and genomes. Nucleic Acids Res 2010; 38:Database issue D234 - 6; http://dx.doi.org/10.1093/nar/gkp874; PMID: 19846593
- Roberts RJ, Belfort M, Bestor T, Bhagwat AS, Bickle TA, Bitinaite J, et al. A nomenclature for restriction enzymes, DNA methyltransferases, homing endonucleases and their genes. Nucleic Acids Res 2003; 31:1805 - 12; http://dx.doi.org/10.1093/nar/gkg274; PMID: 12654995
- Labrie SJ, Samson JE, Moineau S. Bacteriophage resistance mechanisms. Nat Rev Microbiol 2010; 8:317 - 27; http://dx.doi.org/10.1038/nrmicro2315; PMID: 20348932
- Chen Q, Fischer JR, Benoit VM, Dufour NP, Youderian P, Leong JM. In vitro CpG methylation increases the transformation efficiency of Borrelia burgdorferi strains harboring the endogenous linear plasmid lp56. J Bacteriol 2008; 190:7885 - 91; http://dx.doi.org/10.1128/JB.00324-08; PMID: 18849429
- Groot MN, Nieboer F, Abee T. Enhanced transformation efficiency of recalcitrant Bacillus cereus and Bacillus weihenstephanensis isolates upon in vitro methylation of plasmid DNA. Appl Environ Microbiol 2008; 74:7817 - 20; http://dx.doi.org/10.1128/AEM.01932-08; PMID: 18952874
- Monk IR, Shah IM, Xu M, Tan M-W, Foster TJ. (2012) Transforming the Untransformable: Application of Direct Transformation To Manipulate Genetically Staphylococcus aureus and Staphylococcus epidermidis. MBio 3. Available: http://www.ncbi.nlm.nih.gov/pubmed/22434850.
- Zhang G, Wang W, Deng A, Sun Z, Zhang Y, Liang Y, et al. A mimicking-of-DNA-methylation-patterns pipeline for overcoming the restriction barrier of bacteria. PLoS Genet 2012; 8:e1002987; http://dx.doi.org/10.1371/journal.pgen.1002987; PMID: 23028379
- O’Connell Motherway M, O’Driscoll J, Fitzgerald GF, Van Sinderen D. Overcoming the restriction barrier to plasmid transformation and targeted mutagenesis in Bifidobacterium breve UCC2003. Microb Biotechnol 2009; 2:321 - 32; http://dx.doi.org/10.1111/j.1751-7915.2008.00071.x; PMID: 21261927
- Yasui K, Kano Y, Tanaka K, Watanabe K, Shimizu-Kadota M, Yoshikawa H, et al. Improvement of bacterial transformation efficiency using plasmid artificial modification. Nucleic Acids Res 2009; 37:e3; http://dx.doi.org/10.1093/nar/gkn884; PMID: 19004868
- Pokusaeva K, O’Connell-Motherway M, Zomer A, Macsharry J, Fitzgerald GF, van Sinderen D. Cellodextrin utilization by bifidobacterium breve UCC2003. Appl Environ Microbiol 2011; 77:1681 - 90; http://dx.doi.org/10.1128/AEM.01786-10; PMID: 21216899
- O’Connell Motherway M, Zomer A, Leahy SC, Reunanen J, Bottacini F, Claesson MJ, et al. Functional genome analysis of Bifidobacterium breve UCC2003 reveals type IVb tight adherence (Tad) pili as an essential and conserved host-colonization factor. Proc Natl Acad Sci U S A 2011; 108:11217 - 22; http://dx.doi.org/10.1073/pnas.1105380108; PMID: 21690406
- Fanning S, Hall LJ, Cronin M, Zomer A, MacSharry J, Goulding D, et al. Bifidobacterial surface-exopolysaccharide facilitates commensal-host interaction through immune modulation and pathogen protection. Proc Natl Acad Sci U S A 2012; 109:2108 - 13; http://dx.doi.org/10.1073/pnas.1115621109; PMID: 22308390
- Alvarez-Martin P, Fernández M, O’Connell-Motherway M, O’Connell KJ, Sauvageot N, Fitzgerald GF, et al. A conserved two-component signal transduction system controls the response to phosphate starvation in Bifidobacterium breve UCC2003. Appl Environ Microbiol 2012; 78:5258 - 69; http://dx.doi.org/10.1128/AEM.00804-12; PMID: 22635988
- Alvarez-Martin P, O’Connell Motherway M, Turroni F, Foroni E, Ventura M, van Sinderen D. A two-component regulatory system controls autoregulated serpin expression in Bifidobacterium breve UCC2003. Appl Environ Microbiol 2012; 78:7032 - 41; http://dx.doi.org/10.1128/AEM.01776-12; PMID: 22843530
- Riedel CU, Foata F, Goldstein DR, Blum S, Eikmanns BJ. Interaction of bifidobacteria with Caco-2 cells-adhesion and impact on expression profiles. Int J Food Microbiol 2006; 110:62 - 8; http://dx.doi.org/10.1016/j.ijfoodmicro.2006.01.040; PMID: 16713644
- Preising J, Philippe D, Gleinser M, Wei H, Blum S, Eikmanns BJ, et al. Selection of bifidobacteria based on adhesion and anti-inflammatory capacity in vitro for amelioration of murine colitis. Appl Environ Microbiol 2010; 76:3048 - 51; http://dx.doi.org/10.1128/AEM.03127-09; PMID: 20228095
- Gleinser M, Grimm V, Zhurina D, Yuan J, Riedel CU. Improved adhesive properties of recombinant bifidobacteria expressing the Bifidobacterium bifidum-specific lipoprotein BopA. Microb Cell Fact 2012; 11:80; http://dx.doi.org/10.1186/1475-2859-11-80; PMID: 22694891
- Riedel C-U, Foata F, Philippe D, Adolfsson O, Eikmanns B-J, Blum S. Anti-inflammatory effects of bifidobacteria by inhibition of LPS-induced NF-kappaB activation. World J Gastroenterol 2006; 12:3729 - 35; PMID: 16773690
- Philippe D, Heupel E, Blum-Sperisen S, Riedel CU. Treatment with Bifidobacterium bifidum 17 partially protects mice from Th1-driven inflammation in a chemically induced model of colitis. Int J Food Microbiol 2011; 149:45 - 9; http://dx.doi.org/10.1016/j.ijfoodmicro.2010.12.020; PMID: 21257218
- Zhurina D, Zomer A, Gleinser M, Brancaccio VF, Auchter M, Waidmann MS, et al. Complete genome sequence of Bifidobacterium bifidum S17. J Bacteriol 2011; 193:301 - 2; http://dx.doi.org/10.1128/JB.01180-10; PMID: 21037011
- Yu BJ, Kang KH, Lee JH, Sung BH, Kim MS, Kim SC. Rapid and efficient construction of markerless deletions in the Escherichia coli genome. Nucleic Acids Res 2008; 36:e84; http://dx.doi.org/10.1093/nar/gkn359; PMID: 18567910
- Riedel CU, Casey PG, Mulcahy H, O’Gara F, Gahan CGM, Hill C. Construction of p16Slux, a novel vector for improved bioluminescent labeling of gram-negative bacteria. Appl Environ Microbiol 2007; 73:7092 - 5; http://dx.doi.org/10.1128/AEM.01394-07; PMID: 17766445
- MacNeil DJ, Gewain KM, Ruby CL, Dezeny G, Gibbons PH, MacNeil T. Analysis of Streptomyces avermitilis genes required for avermectin biosynthesis utilizing a novel integration vector. Gene 1992; 111:61 - 8; http://dx.doi.org/10.1016/0378-1119(92)90603-M; PMID: 1547955
- Klijn A, Moine D, Delley M, Mercenier A, Arigoni F, Pridmore RD. Construction of a reporter vector for the analysis of Bifidobacterium longum promoters. Appl Environ Microbiol 2006; 72:7401 - 5; http://dx.doi.org/10.1128/AEM.01611-06; PMID: 16997985
- MacConaill LE, Fitzgerald GF, Van Sinderen D. Investigation of protein export in Bifidobacterium breve UCC2003. Appl Environ Microbiol 2003; 69:6994 - 7001; http://dx.doi.org/10.1128/AEM.69.12.6994-7001.2003; PMID: 14660341
- O’Connell Motherway M, Fitzgerald GF, Neirynck S, Ryan S, Steidler L, van Sinderen D. Characterization of ApuB, an extracellular type II amylopullulanase from Bifidobacterium breve UCC2003. Appl Environ Microbiol 2008; 74:6271 - 9; http://dx.doi.org/10.1128/AEM.01169-08; PMID: 18689518
- Law J, Buist G, Haandrikman A, Kok J, Venema G, Leenhouts K. A system to generate chromosomal mutations in Lactococcus lactis which allows fast analysis of targeted genes. J Bacteriol 1995; 177:7011 - 8; PMID: 8522504
- Monk IR, Gahan CGM, Hill C. Tools for functional postgenomic analysis of listeria monocytogenes. Appl Environ Microbiol 2008; 74:3921 - 34; http://dx.doi.org/10.1128/AEM.00314-08; PMID: 18441118
- Turnbough CL Jr., Switzer RL. Regulation of pyrimidine biosynthetic gene expression in bacteria: repression without repressors. Microbiol Mol Biol Rev 2008; 72:266 - 300; http://dx.doi.org/10.1128/MMBR.00001-08; PMID: 18535147
- Weidenmaier C, Peschel A. Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nat Rev Microbiol 2008; 6:276 - 87; http://dx.doi.org/10.1038/nrmicro1861; PMID: 18327271
- Op den Camp HJ, Veerkamp JH, Oosterhof A, Van Halbeek H. Structure of the lipoteichoic acids from Bifidobacterium bifidum spp. pennsylvanicum. Biochim Biophys Acta 1984; 795:301 - 13; http://dx.doi.org/10.1016/0005-2760(84)90080-8; PMID: 6477947
- Fischer W. ‘Lipoteichoic acid’ of Bifidobacterium bifidum subspecies pennsylvanicum DSM 20239. A lipoglycan with monoglycerophosphate side chains. Eur J Biochem 1987; 165:639 - 46; http://dx.doi.org/10.1111/j.1432-1033.1987.tb11488.x; PMID: 3595606
- Janusz MJ, Esser RE, Schwab JH. In vivo degradation of bacterial cell wall by the muralytic enzyme mutanolysin. Infect Immun 1986; 52:459 - 67; PMID: 3516873
- Stimpson SA, Lerch RA, Cleland DR, Yarnall DP, Clark RL, Cromartie WJ, et al. Effect of acetylation on arthropathic activity of group A streptococcal peptidoglycan-polysaccharide fragments. Infect Immun 1987; 55:16 - 23; PMID: 3539800