Abstract
Due to the limited self-renewal capacity of cardiomyocytes, the mammalian heart exhibits impaired regeneration and insufficient ability to restore heart function after injury. Cardiovascular tissue engineering is currently considered as a promising alternative therapy to restore the structure and function of the failing heart. Recent evidence suggests that the epicardium may play critical roles in regulation of myocardial development and regeneration. One of the mechanisms that has been proposed for the restorative effect of the epicardium is the specific physiomechanical cues that this layer provides to the cardiac cells. In this article we explore whether a new generation of epicardium-mimicking, acellular matrices can be utilized to enhance cardiac healing after injury. The matrix consists of a dense collagen scaffold with optimized biomechanical properties approaching those of embryonic epicardium. Grafting the epicardial patch onto the ischemic myocardium—promptly after the incidence of infarct—resulted in preserved contractility, attenuated ventricular remodeling, diminished fibrosis, and vascularization within the injured tissue in the adult murine heart.
Despite remarkable recent medical advances over the past 80 years, including catheter-based, anti-stenosis methods,Citation1,Citation2 contractile-enhancing drugs,Citation1,Citation3,Citation4 and transplantation,Citation1,Citation5,Citation6 heart disease and stroke are still leading causes of death around the world.Citation7 As reported by the American Heart Association, by 2030, 40% of US adults—upwards of 116 million people—will be suffering from cardiovascular diseases.Citation8,Citation9 Cardiovascular-related disease costs will triple between 2010 and 2030 to more than $800 billion a year. Patients who survive acute MI are left with damaged ventricles, prone to scar formation and aneurysmal thinning, which often lead to heart failure.Citation10 This is mainly due to the intrinsic inability of the damaged cardiac tissue to repair after injury.Citation10-Citation13
Given the paucity of donors for heart transplants, current clinical therapies to treat acute heart injuries have shown modest success.Citation14-Citation16 Interventional cardiology for acute myocardial infarction (MI) have yielded significant advances over the past two decades, while there is still a considerable number of patients who either arrive too late to the clinics or are resistant to angioplasty.Citation1 Thus, there is a growing interest in developing new approaches to treat MI. Cardiovascular tissue engineering is currently considered as a promising alternative therapy to restore the structure and function of infarcted adult myocardium via application of a biological substitute (i.e., cardiac patch), onto the ischemic tissue.Citation17-Citation19 To date, few attempts have been made to produce commercially available cardiac patches, mainly by utilizing naturally-occurring matrices (decellularized tissues).Citation20 However, the patch success has been limited primarily due to the poor control on the ultrastructure and physiomechanical properties of the matrix and therefore, mismatch of the patch-host tissue properties, inflammatory response, insufficient vascularization, and risk of thrombosis.Citation21-Citation24
We recently evaluated the epicardial delivery of a novel generation of acellular biomaterial matrices, consisting of an in vitro reconstituted collagen type I scaffold with optimized physiomechanical properties, to treat acute transmural MI in the adult mice.Citation25 Highly hydrated collagen gels were produced from rat tail collagen solution,Citation26-Citation28 and underwent plastic compression (PC) via application of a static compressive stress of 1400 N/m2 for 5 min. PC generated dense sheets of collagen scaffolds with elastic moduli (3–10 kPa) approaching that of the embryonic epicardium (measured by atomic force microscopy). One of the key parameters in maintaining cardiac cells and regulating their proliferation and/or differentiation in the heart’s microenvironment is the stiffness (elasticity) of the extracellular matrix (ECM).Citation17,Citation29-Citation32 Collagen gel stiffness values obtained by PC were within the reported optimal range of substrate elasticity to achieve maximum myocyte contractility and development of immature cardiomyocytes.Citation32
In order to assess compatibility of the collagen scaffolds produced in this study with cardiac cells, we performed in vitro culture of a number of cardiac cells within the gels which underwent PC using different levels of compressive stress: 0, 700, 1000, or 1400 N/m2 for 2–5 min (named: PC0, PC2, PC3, and PCF, respectively, see refs. Citation25,Citation27 for details). We seeded epicardial mesothelial cells (EMC) within collagen gels at a density of 75 000 cells/ml () and evaluated their viability and growth using Alamar Blue™ assayCitation27 under diverse PC. At day 1 in culture, EMCs showed significantly higher viability in non-compressed collagen gels (PC0) when compared with compressed matrices. In this initial stage of culture, viability decreased as the level of plastic compression increased (PC2 to PCF) (). This is in contrast to their longer-term maintenance, as on day 7, matrices with highest levels of compression (PC3 and PCF) showed significantly (P < 0.05) higher cell viability and growth when compared with non-compressed (PC0) matrices (). On day 14, cell metabolism in all gel specimens reached a plateau (no significant difference).
Figure 1. In vitro culture of epicardial mesothelial cell (EMC) line seeded within various collagen matrices at a density of 75 000 cells/ml. Collagen gels underwent different levels of plastic compression: non-compressed (PC0), partially compressed (PC2 and PC3), and fully compressed (PCF) (for details please see ref. Citation25). (A) Metabolic activity of EMCs in collagen matrices was assessed on days 1, 7, and 14 by measuring Alamar Blue™ reduction. On the contrary to day 1, on days 7 and 14 epicardial cells exhibited the lowest metabolic activity within non-compressed gel specimens (PC0) when compared with the various compressed gels at each time point. In all gel specimens, cell viability and/or growth increased significantly from day 1 to 14. *: significantly different compared with non-compressed gel, at the same time point. (B) EMC-induced contraction of collagen matrices over 15 d of culture. Top: schematic demonstration of the procedure used to quantify global matrix contraction. Bottom: measured contraction for acellular gels (control), PC0, PC2, PC3, and PCF. In contrast to compressed cellular matrices, PC0 exhibited a rapid decline in surface area within the first days of culture, followed by a gradual increase in contraction over time. Increase in the level of compression, from PC2 to PCF resulted in significant decrease in contraction rate. Contraction was not evident for PCF (until after day 11) and for acellular matrices. Statistical analysis was performed using one-way ANOVA (P < 0.05).
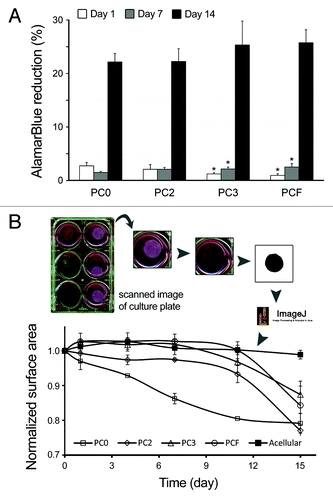
In addition to viability, we analyzed cell-induced contraction of collagen matrices by culturing EMCs in PC0 to PCF gels and measuring their surface area at indicated time points (). Surface area was measured via scanning the gels using a Canon Scanner at 300 dpi resolution and quantification using ImageJ. In analogy to our previous reports for other cell types including bone marrow-derived mesenchymal stem cells and skin fibroblasts,Citation27,Citation33 EMCs yielded significantly greater levels of matrix contraction in PC0 gels throughout the 15-day culture. Cell-induced contraction was significantly lower in matrices with greater level of compression (PC2 to PCF). PCF gels exhibited no detectable contraction until day 11, similar to acellular gels used as control (). Reduced contraction in more compressed matrices can be attributed to improved mechanical properties (elastic moduli) of collagen gels as result of PC.Citation25,Citation27,Citation33 Cell viability and contraction results together, demonstrate that plastic compression of collagen gels has no adverse effect on cell viability and growth. On the contrary, PC improves cell viability and also diminishes collagen matrix remodeling by cells in long-term culture; hence, suggesting that PCF—which was used in this study as cardiac patch—can provide an optimal, cell-friendly microenvironment for cardiac cells in vivo.Citation25,Citation27,Citation33
“The relatively slower growth rate of EMCs in the matrices within the first 7 days of culture (AlamarBlue result, ) can be well corresponded to the 7-9 days of delay in EMC-induced matric contraction as shown in . Thereupon, delayed onset of matrix contraction might be partly related to the lower EMC proliferation rate in the matrices. This lag phase in cells 3D culture within matrices has been previously reported by usCitation27,Citation33 as well as other researchers.Citation34,Citation35 Cell-induced contraction of matrices might pose substantial technical issues (e.g., significant changes in graft size or shape) to the application of scaffolds for tissue repair in the in vivo models. The 7- to 9-day lag phase in the culture of cells within 3D compressed scaffolds can therefore provide an opportunity to avoid and/or reduce graft deformation pre- and shortly after implantationCitation27.” “Whether this lag phase also occurs in the in vivo experiments, when native cardiac cells migrate extensively into the patch,Citation25 and possible correspondence of the delay phase to tissue regeneration are questions that need to be addressed in the future studies.”
We next implanted the engineered patches onto the infarcted myocardium in the left ventricle (LV) of male mouse heart. A left thoracotomy was performed to ligate the left anterior descending artery. This was immediately followed by suturing the collagen patch onto the surface of the ischemic myocardium (at two points). Echocardiography conducted pre-surgery (baseline) and at weeks 2 and 4 post MI showed significant improvement in heart contractility (ejection fraction and fractional shortening), inhibition of cardiac remodeling, i.e., reduction in LV internal diameter and increase in LV posterior wall thickness in the mouse hearts treated with epicardial patch, when compared with MI with no treatment.Citation25 Hence, the bioengineered patch exerted beneficial in vivo effects on cardiac function and remodeling following MI.
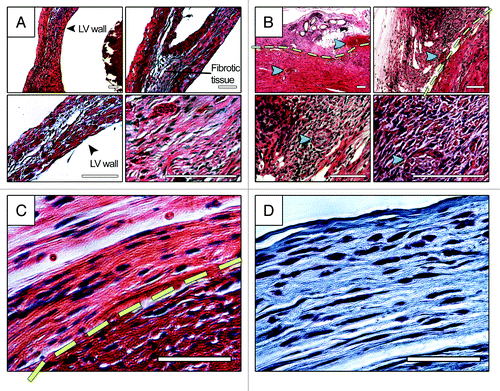
Histology analysis of heart sections four weeks post grafting demonstrated secure attachment of the graft to the host myocardium, extensive migration of native cardiac cells into the patch, as well as a significant number of new functional blood vessels within the infarct (). In comparison with the MI hearts with no treatment, epicardial delivery of the patch also resulted in diminished fibrosis and LV remodeling (dilation and wall thinning). Interestingly, in some areas, migratory cardiac cells within the patch aligned themselves with collagen fibrils (). Furthermore, immunostaining analysis demonstrated a significantly greater number of α-smooth muscle actin+ cells as well as a small population of α-actinin+ (immature-like) myocytes with disturbed, unorganized sarcomeric pattern within the infarct in the hearts treated with patch, suggesting enhanced angiogenesis and either proliferation or migration of muscle cells into the lesion. This is while no significant immune response was detected as a result of epicardial patch treatment.
Figure 2. Histology analysis of infarcted mouse hearts with no treatment (A) and treated with patch (B-D), four weeks post implantation. (A) and (B) show HE staining of heart sections at four different magnifications. (C) and (D) show HE and trichrome staining of patch-treated heart sections, respectively. Extensive fibrosis can be seen in MI heart with no treatment. Endogenous cardiac cells migrated to the patch and, in some sections, aligned themselves with collagen fibrils, (C)and(D). Yellow line in panels (B) and (C) demonstrate the approximate boundary between the patch and the host tissue. Green arrows point to the blood vessel with blood stream inside the patch and at the border zone. Blue arrows show the suture spot within the patch which has been replaced by cellularized tissue. Scale bars in panels (A) and (B) are 100 µm. Scale bars in panels (C) and (D) are 50 µm.
Although, the application of various cardiac patches, immediately following the injury, has been reported in a number of studies (e.g. refs. Citation36,Citation37), the clinical relevance of such models in terms of recapitulating the current therapeutic interventions is elusive. The simultaneous incidence of injury and patch implantation is an intrinsic technical limitation associated with the mouse model, which is not tolerant to multiple invasive interventions. We are currently evaluating the patch function in mouse and pig models of heart ischemic injury, in which, identical patches are being grafted onto the injured myocardium one week after the incidence of the infarct. These latter studies are believed to offer greater potential in terms of clinical translation.Citation18,Citation38
In summary, this article introduced a novel cardiac patch system which provides physiomechanical cues resembling those of the epicardial tissue in the embryonic stage. By exposing native cardiac cells to an ECM which recapitulates the dynamic stage of heart development when cardiomyocytes still proliferate, engineered epicardial graft aids to repair the adult heart tissue following acute injuries, independently from exogenous cells or other factors. Other mechanisms that can contribute to patch function include: providing a temporary mechanical support to the ischemic tissue with poor mechanical integrity, easing cell migration, proliferation, and angiogenesis in a biomimetic microenvironment, and partial preservation of the heart muscle cells within the infarct and the tissue beneath the patch. Further investigations on potential applications of the engineered patches as local, controlled-delivery devices to supply the damaged heart tissue with various therapeutic factors and/or cells can usher in a new wave of clinical therapies for the adult heart repair.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
References
- Topol EJ. Current status and future prospects for acute myocardial infarction therapy. Circulation 2003; 108:Suppl 1 III6 - 13; http://dx.doi.org/10.1161/01.CIR.0000086950.37612.7b; PMID: 14605014
- Marso SP, Steg G, Plokker T, Holmes D, Park SJ, Kosuga K, Tamai H, Macaya C, Moses J, White H, et al. Catheter-based reperfusion of unprotected left main stenosis during an acute myocardial infarction (the ULTIMA experience). Unprotected Left Main Trunk Intervention Multi-center Assessment. Am J Cardiol 1999; 83:1513 - 7; http://dx.doi.org/10.1016/S0002-9149(99)00139-3; PMID: 10363863
- Makdisse MRP, Matsushita A de M, Goncalves I Jr., Miranda O, Gomes AC, Cartocci MM, Covre S, Carvalho AC de C. Pharmacological therapy for myocardial infarction in the elderly: An 8-year analysis. Arq Bras Cardiol 2002; 78:364 - 73; http://dx.doi.org/10.1590/S0066-782X2002000400003; PMID: 12011952
- Shannon AW, Harrigan RA. General pharmacologic treatment of acute myocardial infarction. Emerg Med Clin North Am 2001; 19:417 - 31; http://dx.doi.org/10.1016/S0733-8627(05)70192-9; PMID: 11373987
- Lee MS, Lill M, Makkar RR. Stem cell transplantation in myocardial infarction. Rev Cardiovasc Med 2004; 5:82 - 98; PMID: 15184842
- Fassa A-A, Himbert D, Vahanian A. Transcatheter aortic valve replacement: current application and future directions. Curr Cardiol Rep 2013; 15:353; http://dx.doi.org/10.1007/s11886-013-0353-7; PMID: 23420448
- The Heart and Stroke Foundation. Statistics Canada - Mortality, summary list of causes 2008. 2011;
- Lloyd-Jones. Heart Disease and Stroke Statistics-2009 Update: A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee (vol 119, pg e21, 2009). Circulation 2010; 122:E11 - 11
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al, American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation 2012; 125:188 - 97; http://dx.doi.org/10.1161/CIR.0b013e3182456d46; PMID: 22215894
- Gaziano TA. Cardiovascular disease in the developing world and its cost-effective management. Circulation 2005; 112:3547 - 53; http://dx.doi.org/10.1161/CIRCULATIONAHA.105.591792; PMID: 16330695
- Mercola M, Ruiz-Lozano P, Schneider MD. Cardiac muscle regeneration: lessons from development. Genes Dev 2011; 25:299 - 309; http://dx.doi.org/10.1101/gad.2018411; PMID: 21325131
- Steinhauser ML, Lee RT. Regeneration of the heart. EMBO Mol Med 2011; 3:701 - 12; http://dx.doi.org/10.1002/emmm.201100175; PMID: 22095736
- Laflamme MA, Murry CE. Heart regeneration. Nature 2011; 473:326 - 35; http://dx.doi.org/10.1038/nature10147; PMID: 21593865
- Malliaras K, Kreke M, Marbán E. The stuttering progress of cell therapy for heart disease. Clin Pharmacol Ther 2011; 90:532 - 41; http://dx.doi.org/10.1038/clpt.2011.175; PMID: 21900888
- Gho JMIH, Kummeling GJM, Koudstaal S, Jansen Of Lorkeers SJ, Doevendans PA, Asselbergs FW, Chamuleau SAJ. Cell therapy, a novel remedy for dilated cardiomyopathy? A systematic review. J Card Fail 2013; 19:494 - 502; http://dx.doi.org/10.1016/j.cardfail.2013.05.006; PMID: 23834925
- Takehara N. Cell therapy for cardiovascular regeneration. Ann Vasc Dis 2013; 6:137 - 44; http://dx.doi.org/10.3400/avd.ra.13.00019; PMID: 23825492
- Curtis MW, Russell B. Cardiac tissue engineering. J Cardiovasc Nurs 2009; 24:87 - 92; http://dx.doi.org/10.1097/01.JCN.0000343562.06614.49; PMID: 19125130
- Cui J, Li J, Mathison M, Tondato F, Mulkey SP, Micko C, Chronos NA, Robinson KA. A clinically relevant large-animal model for evaluation of tissue-engineered cardiac surgical patch materials. Cardiovasc Revasc Med 2005; 6:113 - 20; http://dx.doi.org/10.1016/j.carrev.2005.07.006; PMID: 16275607
- Ozawa T, Mickle DA, Weisel RD, Koyama N, Ozawa S, Li RK. Optimal biomaterial for creation of autologous cardiac grafts. Circulation 2002; 106:Suppl 1 I176 - 82; PMID: 12354729
- Badylak SF, Taylor D, Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng 2011; 13:27 - 53; http://dx.doi.org/10.1146/annurev-bioeng-071910-124743; PMID: 21417722
- Smart N, Bollini S, Dubé KN, Vieira JM, Zhou B, Davidson S, Yellon D, Riegler J, Price AN, Lythgoe MF, et al. De novo cardiomyocytes from within the activated adult heart after injury. Nature 2011; 474:640 - 4; http://dx.doi.org/10.1038/nature10188; PMID: 21654746
- Vunjak-Novakovic G, Tandon N, Godier A, Maidhof R, Marsano A, Martens TP, Radisic M. Challenges in cardiac tissue engineering. Tissue Eng Part B Rev 2010; 16:169 - 87; http://dx.doi.org/10.1089/ten.teb.2009.0352; PMID: 19698068
- Venugopal JR, Prabhakaran MP, Mukherjee S, Ravichandran R, Dan K, Ramakrishna S. Biomaterial strategies for alleviation of myocardial infarction. J R Soc Interface 2012; 9:1 - 19; http://dx.doi.org/10.1098/rsif.2011.0301; PMID: 21900319
- Jawad H, Ali NN, Lyon AR, Chen QZ, Harding SE, Boccaccini AR. Myocardial tissue engineering: a review. J Tissue Eng Regen Med 2007; 1:327 - 42; http://dx.doi.org/10.1002/term.46; PMID: 18038427
- Serpooshan V, Zhao M, Metzler SA, Wei K, Shah PB, Wang A, Mahmoudi M, Malkovskiy AV, Rajadas J, Butte MJ, et al. The effect of bioengineered acellular collagen patch on cardiac remodeling and ventricular function post myocardial infarction. Biomaterials 2013; 34:9048 - 55; http://dx.doi.org/10.1016/j.biomaterials.2013.08.017; PMID: 23992980
- Serpooshan V, Quinn TM, Muja N, Nazhat SN. Hydraulic permeability of multilayered collagen gel scaffolds under plastic compression-induced unidirectional fluid flow. Acta Biomater 2013; 9:4673 - 80; http://dx.doi.org/10.1016/j.actbio.2012.08.031; PMID: 22947324
- Serpooshan V, Julien M, Nguyen O, Wang H, Li A, Muja N, Henderson JE, Nazhat SN. Reduced hydraulic permeability of three-dimensional collagen scaffolds attenuates gel contraction and promotes the growth and differentiation of mesenchymal stem cells. Acta Biomater 2010; 6:3978 - 87; http://dx.doi.org/10.1016/j.actbio.2010.04.028; PMID: 20451675
- Brown RA, Wiseman M, Chuo CB, Cheema U, Nazhat SN. Ultrarapid engineering of biomimetic materials and tissues: Fabrication of nano- and microstructures by plastic compression. Adv Funct Mater 2005; 15:1762 - 70; http://dx.doi.org/10.1002/adfm.200500042
- Arshi A, Nakashima Y, Nakano H, Eaimkhong S, Evseenko D, Reed J, Stieg AZ, Gimzewski JK, Nakano A. Rigid microenvironments promote cardiac differentiation of mouse and human embryonic stem cells. Sci Technol Adv Mater 2013; 14:025003; http://dx.doi.org/10.1088/1468-6996/14/2/025003; PMID: 24311969
- Rehfeldt F, Engler AJ, Eckhardt A, Ahmed F, Discher DE. Cell responses to the mechanochemical microenvironment--implications for regenerative medicine and drug delivery. Adv Drug Deliv Rev 2007; 59:1329 - 39; http://dx.doi.org/10.1016/j.addr.2007.08.007; PMID: 17900747
- Reilly GC, Engler AJ. Intrinsic extracellular matrix properties regulate stem cell differentiation. J Biomech 2010; 43:55 - 62; http://dx.doi.org/10.1016/j.jbiomech.2009.09.009; PMID: 19800626
- Engler AJ, Carag-Krieger C, Johnson CP, Raab M, Tang HY, Speicher DW, Sanger JW, Sanger JM, Discher DE. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J Cell Sci 2008; 121:3794 - 802; http://dx.doi.org/10.1242/jcs.029678; PMID: 18957515
- Serpooshan V, Muja N, Marelli B, Nazhat SN. Fibroblast contractility and growth in plastic compressed collagen gel scaffolds with microstructures correlated with hydraulic permeability. J Biomed Mater Res A 2011; 96:609 - 20; http://dx.doi.org/10.1002/jbm.a.33008; PMID: 21268235
- Nirmalanandhan VS, Levy MS, Huth AJ, Butler DL. Effects of cell seeding density and collagen concentration on contraction kinetics of mesenchymal stem cell-seeded collagen constructs. Tissue Eng 2006; 12:1865 - 72; http://dx.doi.org/10.1089/ten.2006.12.1865; PMID: 16889516
- Selezneva II, Savintseva IV, Vikhlyantseva EF, Davydova GA, Gavrilyuk BK. Immobilization and long-term culturing of mouse embryonic stem cells in collagen-chitosan gel matrix. Bull Exp Biol Med 2006; 142:119 - 22; http://dx.doi.org/10.1007/s10517-006-0308-8; PMID: 17369920
- Miyagi Y, Chiu LLY, Cimini M, Weisel RD, Radisic M, Li R-K. Biodegradable collagen patch with covalently immobilized VEGF for myocardial repair. Biomaterials 2011; 32:1280 - 90; http://dx.doi.org/10.1016/j.biomaterials.2010.10.007; PMID: 21035179
- Ozawa T, Mickle DAG, Weisel RD, Koyama N, Wong H, Ozawa S, Li R-K. Histologic changes of nonbiodegradable and biodegradable biomaterials used to repair right ventricular heart defects in rats. J Thorac Cardiovasc Surg 2002; 124:1157 - 64; http://dx.doi.org/10.1067/mtc.2002.127449; PMID: 12447182
- Fujimoto KL, Tobita K, Guan J, Hashizume R, Takanari K, Alfieri CM, Yutzey KE, Wagner WR. Placement of an elastic biodegradable cardiac patch on a subacute infarcted heart leads to cellularization with early developmental cardiomyocyte characteristics. J Card Fail 2012; 18:585 - 95; http://dx.doi.org/10.1016/j.cardfail.2012.05.006; PMID: 22748493
