Introduction
RNA interference (RNAi) is a cellular pathway preserved in eukaryotic cells of animals, plants and fungi. In the RNAi process, double-stranded small interfering RNA (siRNA) is recognized by the RNA-induced silencing complex (RISC). The antisense strand of siRNA binds to the mRNA that are homologous to the siRNA sequence and induces the degradation of the target mRNA with the help of argonaute 2 (Ago2) protein in RISC, ultimately resulting in the inhibition of the mRNA translation.Citation1 The principle of RNAi was first employed to downregulate the target gene in mammalian cells by Tuschl et al. in 2001.Citation2 Since then employing synthetic siRNAs to silence targeted genes has been intensively investigated for uses as a powerful tool in laboratory studiesCitation3,Citation4 as well as in RNAi-based therapies.Citation5-Citation7
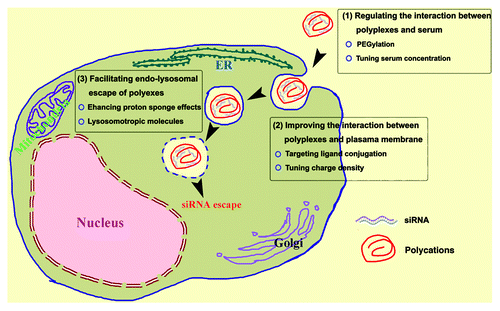
Delivery vehicles for siRNA are crucial to guarantee an effective RNAi, which prevent siRNAs from degradation and deliver them into the cell as well. However, taken up by cells is far from enough for the effective RNAi if delivered siRNAs fail to be localized at the cytoplasm where RISC is staying.Citation3 Viral vector systems include adeno-associated viruses and lentiviruses. There are now many examples of the use of viral vector-mediated RNAi to inhibit gene expression in animal models of disease, and in many cases proof-of-principle has been demonstrated.Citation8 However, a number of concerns have raised questions regarding the clinical application of this technology, including off-target effects, risks of immunogenicity and potential carcinogenicity.Citation9 Hence nonviral vectors, particularly synthetic cationic polymers, have been attracting ever-growing interests due to facile synthesis, controllable modification and convenient manipulation,Citation10 only a simple mixing of the cationic polymers and siRNAs allow the formation of polyplexes. However, two crucial issues are raising major challenges to the cationic polymeric carriers: (1) Although polyplexes can be steadily internalized by various kinds of cells, the efficiency of lysosomal escape for the siRNA is limited. (2) The cytotoxicity of polyplexes is high, which is attributable to positive charges of the cationic polymers. To overcome these barriers, efforts are mainly placed in three aspects ().
Figure 1. Schematic illustration to strategies of optimizing cationic polymer-mediated RNAi: (1) regulating the interaction between polyplexes and the serum in the biological environment to modulate the particles size for efficient internalization and low cytotoxicity; (2) improving the interaction between polyplexes and the cell membrane to increase targeting endocytosis; (3) facilitating the endo-lysosomal escape of polyplexes by stimuli-responsive modification.
Regulating Interactions between Polyplexes and the Serum
Zeng’s group proposed a novel cationic copolymer platform for siRNA delivery in a safe and efficient way,Citation11 they reported three block copolymers including PEG-PLL, PLL-PEG-PLL, and PLL-PPG-PEG-PPG-PLL. The PEG and PPG are used for improving the biocompatibility and modulating the size of the polyplexes, which allows them efficient cellular internalization as well as low cytotoxicity. In the serum-containing culture medium the gene silence mediated by PLL-PPG-PEG-PPG-PLL is comparable to that mediated by Lipofectamine 2000. This is attractive because serum can significantly compromise the RNAi efficiency mediated by cationic polymers due to the nonspecific adsorption of proteins, while serum-free condition is not only toxic to the cells, but also not available in biological environment, particularly in blood. The high RNAi efficiency should be attributed to the PEG and PPG segments, for they are able to repel the protein adherence. We speculate that the PEG and PPG chain segments may partly locate on the surface and partly in the core of the polyplexes particles. Moreover, the PEGylation strategy can hinder the clearance by RES system in vivo and increase the circulation time of polyplexes in blood.Citation6 Our previous work found that serum free is not always necessary for cationic polymer carriers in the RNAi. The serum at a proper concentration in the culture medium not only modulates the size of polyplexes particles to allow the efficient cellular uptake and lysosomal escape, but also reduces the cytotoxicity of the polycomplex.Citation12
Improving Interactions between Polyplexes and Cell Membranes
The interaction between polyplexes and the cell membrane determines how many siRNAs can be taken up, which is closely associated with the final RNAi efficiency. There are many cellular uptake pathways for cells to internalize polyplexes, mainly including receptor-mediated endocytosis, receptor-independent endocytosis, and phagocytosis.Citation13 In addition to the endocytosis or phagocytosis, in the serum-free medium, a possible alternative pathway for the internalization of polyplexes is direct penetration: strong electrostatic interactions between the positively-charged polymer and the cell membrane may result in pores on the cell membrane, which facilitate the cellular uptake.Citation14 However, pores on the membrane also lead to the leakage of the cellular contents and a significant cytotoxicity. In the serum containing medium, the polyplexes are associated with proteins, under this circumstance, the direct penetration is not available. To enhance the receptor-mediated endocytosis, targeting moieties such as transferrin and folic acids are tethered to polymers, which is a widely applied route.Citation15,Citation16 Tuning the charge densities of the polymer is another potential strategy to increase the uptake of siRNA.Citation17
Facilitating the Endo-Lysosomal Escape of siRNA
After engulfed, most of the polyplexes enter to the endo-lysosomal system. In the process of endosome maturation, the pH value is gradually decreased from the early endosome to the late endosome and to the lysosomes, The efficacy of siRNA molecules deteriorates in the harsh environment of the endo-lysosome. Only those siRNA locating in the cytoplasm and recognized by the RISC can induce RNAi effects, consequently the endo-lysosomal escape of siRNA in polyplexes is a pivotal issue after their uptake.Citation3 The endo-lysosomal escape of polyplexes is largely dependent on “proton sponge” effects.Citation3 Increasing the pH-buffering capability of the polymer carrier would benefit RNAi effects, hence changing the side chain or main chain of polymers is a most employed strategy. For instance, Itaka et al. synthesized PEG-poly(3-[(3-aminopropyl) amino] propylaspartamide (PEG-DPT) with a diamine side chain to buffer the pH in endosome, the resulting RNAi efficiency is higher than that induced by the commercialized reagent RNAiFect.Citation18 Besides modifying the chemical composition of polymers, lysosomotropic molecules such as amphotericin B (AmB) are brought in to assist the endo-lysosomal escape. AmB can increase the permeability of membrane and thus facilitates the endo-lysosomal escape of siRNA. Examples include that Yu et al. employed the polymer PDMA-b-PDPA in combination with AmB and siRNA to realize the enhancement of RNAi effect.Citation19 It should be noted that the promotion of endo-lysosomal escape is accompanied with cytotoxicity, as the rupture or permiablization of lysosomal membrane also lead to the leakage of cathepsins which further mediated the cell death.Citation20
Stimuli-Reactive Polymer in siRNA Delivery
The stimuli-reactive strategy introduces the stimuli-activated block into polymer chains. The stimuli include acidity, enzyme, redox potential and so on.Citation21 For example, the microenvironment of tumor is of low pH value, hypoxemia and overexpression of specific enzymes such as matrix metalloproteinases (MMP). Shim et al. developed a pH-sensitive ketalized PEI to facilitate the cytoplasmic release of siRNA molecules.Citation22 Li et al. reported a MMP-responsive polymer to allow siRNA release when the polyplexes enter the MMP-rich environment. These designs provide a strategy of realizing the enrichment of polyplexes at the target organs and thus improve the RNAi efficiency in vivo.Citation23 The advantage of stimuli-responsive polymers is that they mediate predictable and targetable RNAi .
Perspectives for Polymeric Carrier-Mediated RNAi
Nowadays, more and more polymers are being explored to deliver siRNA though there are many challenges on the way. The core issues of optimizing the performance of polyplexes is to balance the RNAi efficiency and cytotoxicity and settle the targeting issue, all of which are highly correlated with the interaction between polyplexes and cells. A clear demonstration of behaviors and the fate of polyplexes in the cell will guide the optimization of polymeric carriers in siRNA delivery.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Acknowledgments
Authors thank for financial support from National Key Program of China (973 program2011CB933504).
References
- Meister G, Tuschl T. Mechanisms of gene silencing by double-stranded RNA. Nature 2004; 431:343 - 9; http://dx.doi.org/10.1038/nature02873; PMID: 15372041
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001; 411:494 - 8; http://dx.doi.org/10.1038/35078107; PMID: 11373684
- Dominska M, Dykxhoorn DM. Breaking down the barriers: siRNA delivery and endosome escape. J Cell Sci 2010; 123:1183 - 9; http://dx.doi.org/10.1242/jcs.066399; PMID: 20356929
- Koo CX, Fang W, Salto-Tellez M, Leong DT. Coexpressing shRNA with fluorescence tags for quantification of cell migration studies. Mol Biol Rep 2012; 39:7695 - 703; http://dx.doi.org/10.1007/s11033-012-1605-0; PMID: 22350264
- Dorsett Y, Tuschl T. siRNAs: applications in functional genomics and potential as therapeutics. Nat Rev Drug Discov 2004; 3:318 - 29; http://dx.doi.org/10.1038/nrd1345; PMID: 15060527
- Akhtar S, Benter IF. Nonviral delivery of synthetic siRNAs in vivo. J Clin Invest 2007; 117:3623 - 32; http://dx.doi.org/10.1172/JCI33494; PMID: 18060020
- Aagaard L, Rossi JJ. RNAi therapeutics: principles, prospects and challenges. Adv Drug Deliv Rev 2007; 59:75 - 86; http://dx.doi.org/10.1016/j.addr.2007.03.005; PMID: 17449137
- Couto LB, High KA. Viral vector-mediated RNA interference. Curr Opin Pharmacol 2010; 10:534 - 42; http://dx.doi.org/10.1016/j.coph.2010.06.007; PMID: 20620113
- Verma IM, Somia N. Gene therapy -- promises, problems and prospects. Nature 1997; 389:239 - 42; http://dx.doi.org/10.1038/38410; PMID: 9305836
- Park TG, Jeong JH, Kim SW. Current status of polymeric gene delivery systems. Adv Drug Deliv Rev 2006; 58:467 - 86; http://dx.doi.org/10.1016/j.addr.2006.03.007; PMID: 16781003
- Dai Z, Arévalo MT, Li J, Zeng M. Addition of poly (propylene glycol) to multiblock copolymer to optimize siRNA delivery. Bioengineered 2013; 5; PMID: 24424156
- Zhang W, Liu J, Tabata Y, Meng J, Xu H. The effect of serum in culture on RNAi efficacy through modulation of polyplexes size. Biomaterials 2014; 35:567 - 77; http://dx.doi.org/10.1016/j.biomaterials.2013.09.102; PMID: 24120041
- Khalil IA, Kogure K, Akita H, Harashima H. Uptake pathways and subsequent intracellular trafficking in nonviral gene delivery. Pharmacol Rev 2006; 58:32 - 45; http://dx.doi.org/10.1124/pr.58.1.8; PMID: 16507881
- Lin J, Alexander-Katz A. Cell membranes open “doors” for cationic nanoparticles/biomolecules: insights into uptake kinetics. ACS Nano 2013; 7:10799 - 808; http://dx.doi.org/10.1021/nn4040553; PMID: 24251827
- Dohmen C, Edinger D, Fröhlich T, Schreiner L, Lächelt U, Troiber C, Rädler J, Hadwiger P, Vornlocher HP, Wagner E. Nanosized multifunctional polyplexes for receptor-mediated siRNA delivery. ACS Nano 2012; 6:5198 - 208; http://dx.doi.org/10.1021/nn300960m; PMID: 22646997
- Davis ME. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol Pharm 2009; 6:659 - 68; http://dx.doi.org/10.1021/mp900015y; PMID: 19267452
- Suma T, Miyata K, Ishii T, Uchida S, Uchida H, Itaka K, Nishiyama N, Kataoka K. Enhanced stability and gene silencing ability of siRNA-loaded polyion complexes formulated from polyaspartamide derivatives with a repetitive array of amino groups in the side chain. Biomaterials 2012; 33:2770 - 9; http://dx.doi.org/10.1016/j.biomaterials.2011.12.022; PMID: 22200535
- Itaka K, Kanayama N, Nishiyama N, Jang WD, Yamasaki Y, Nakamura K, Kawaguchi H, Kataoka K. Supramolecular nanocarrier of siRNA from PEG-based block catiomer carrying diamine side chain with distinctive pKa directed to enhance intracellular gene silencing. J Am Chem Soc 2004; 126:13612 - 3; http://dx.doi.org/10.1021/ja047174r; PMID: 15493907
- Yu H, Zou Y, Wang Y, Huang X, Huang G, Sumer BD, Boothman DA, Gao J. Overcoming endosomal barrier by amphotericin B-loaded dual pH-responsive PDMA-b-PDPA micelleplexes for siRNA delivery. ACS Nano 2011; 5:9246 - 55; http://dx.doi.org/10.1021/nn203503h; PMID: 22011045
- Repnik U, Stoka V, Turk V, Turk B. Lysosomes and lysosomal cathepsins in cell death. Biochim Biophys Acta 2012; 1824:22 - 33; http://dx.doi.org/10.1016/j.bbapap.2011.08.016; PMID: 21914490
- Shim MS, Kwon YJ. Stimuli-responsive polymers and nanomaterials for gene delivery and imaging applications. Adv Drug Deliv Rev 2012; 64:1046 - 59; http://dx.doi.org/10.1016/j.addr.2012.01.018; PMID: 22329941
- Shim MS, Kwon YJ. Acid-responsive linear polyethylenimine for efficient, specific, and biocompatible siRNA delivery. Bioconjug Chem 2009; 20:488 - 99; http://dx.doi.org/10.1021/bc800436v; PMID: 19199781
- Li H, Yu SS, Miteva M, Nelson CE, Werfel T, Giorgio TD, Duvall CL. Matrix metalloproteinase responsive, proximity-activated polymeric nanoparticles for siRNA delivery. Adv Funct Mater 2013; 23:3040 - 52; http://dx.doi.org/10.1002/adfm.201202215
