Abstract
Engineered alternative skin in all its forms and shapes serve to provide temporary or permanent wound closure such as in the case of partial and full-thickness burns. The need for collagen-based regeneration templates is motivated by the fact that dermal regeneration of full-thickness injuries does not occur spontaneously and is inundated by contraction and scarring. Partial-thickness burns in turn can regress as a result of infection and improper treatment and require appropriate treatment. Nylon-silicone laminates such as Biobrane®, and more recently AWBAT®, address this by serving as a temporary barrier. Enhanced collagen-based scaffolds today, although not perfect, remain invaluable. Our initial approach was to characterize the design considerations and explore the use of collagen in the fabrication of a dermal regeneration matrix and a silicone-nylon bilaminate. Here we expand our initial research on scaffold fabrication and explore possible strategies to improve the outcome of collagen-scaffold medicated wound healing.
Burn treatment and the correction of skin defects remains a challenge. Current treatment approaches rely on engineered skin substitutes or alternative tissue, and these products have become important in the management of partial and full-thickness burns. Products like these aim to restore barrier function, facilitate wound healing, and manage pain and can either be temporary, semi-permanent, or permanent.Citation1,Citation2 Their building blocks can be classified as biological (natural or artificial) or alloplastic, and some employ a combination of both in order to substitute or replace the epidermis, dermis, and both the epidermis and dermis.Citation3-Citation6 The pioneering work of Burke and YannasCitation7,Citation8 and WoodroofCitation9 in the 1980s led to the development of the first commercially available products, Integra and Biobrane® respectively, and thus paved the way for the array of skin substitutes that are available today as summarized in references Citation3–Citation6 and Citation10.
The use of pre-fabricated collagen-based scaffolds will prevail and, although not perfect, they are currently the most successful option.Citation6 Total skin replacement remains out of reach even with the advances in tissue engineering. Cellular based skin substitutes, regardless of the origin of the cells, are dependent on either discreet delivery systems or sophisticated tissue engineering scaffolds. One drawback of this approach is the extensive amount of time required to culture the cells in vitro and allow for the modeling of an extracellular matrix (ECM).Citation11,Citation12 Alterations in the biochemical composition of engineered matrices might hold the key toward improved and accelerated wound healing. Four major scaffolding approaches are currently employed in tissue engineering and include pre-made porous matrices, which include collagen-based scaffolds for partial and full-thickness burns (); tissue cultures and the ECM they form; decellularized ECM (); and 3D cultures in a hyderogel.Citation12
Figure 1. (A) Scanning Electron Microscopy (SEM) image demonstrating randomly distributed thick bundles of collagen (mainly type I) of acellular cadaver dermis (scale bar = 50 μm). (B) SEM of the fabricated collagen-based scaffolds show that they are not perfect copies of normal dermis and characteristically demonstrate large amounts of sheets, fibers and polygonal pores. This matrix was formed by using a 0.6% (w/w) collagen concentration and a controlled freeze rate of 0.92 °C/min (scale bar = 100 μm). (C) and (D) (scale bar = 100 μm): SEM of the nylon-based scaffolds with the epidermal portion (E) viewed from inferior and laterally (average thickness of 195.08 ± 3.70 μm). The bound knitted tri-filaments of the nylon mesh are indicated by the arrows.
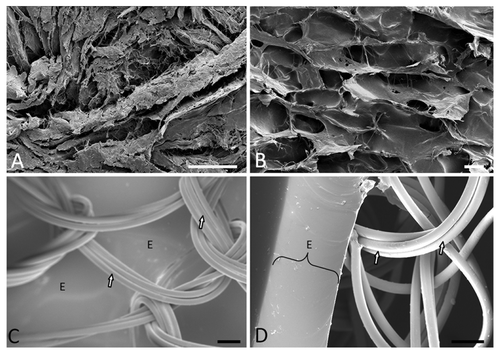
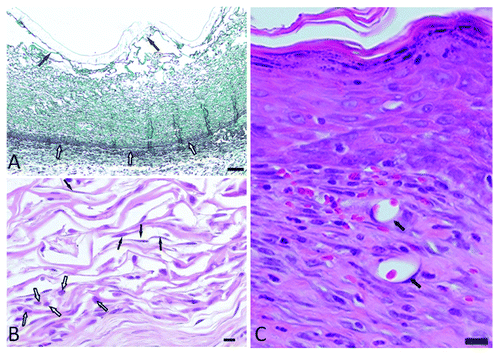
The design considerations and development of pre-made skin replacements are vast and well documented. It is currently accepted that the design considerations should include biocompatibility, controlled biodegradability, low or no antigenicity, a suitable micro-structure or architecture, and resistance to shear forces.Citation6,Citation13,Citation14 The micro-structure relies on the pore structure and size and the total surface area of the scaffold.Citation12,Citation14 The micro-architecture, pore diameter ranging between 20 and 120 µm, and resistance against enzymatic degradation for at least 21 days in vivo allows for neo-collagenesis.Citation14 These attributes can be achieved through tried and tested protocols and can also be manipulated through changes in the collagen concentration, rate of freezing and method of cross-linking.Citation14-Citation19 A typical protocol to construct a collagen-based extracellular matrix relies of the following sequential steps: type I atelocollagen extraction from bovine Achilles tendons, coprecipitate formation with a final collagen:chondroitin-6-sulfate ratio of 92:8, homogenization under controlled temperature, degassing, controlled freezing, lyophilization, dehydrothermal treatment (DHT) at 105 °C and 0.2 mbar for 24 h, silicone coating (such as the use of Dow Corning, Silastic®, Q7-4840), chemical cross-linking, and final washing step.Citation14-Citation18 Highly porous matrices were obtained through the use of a 0.6% (w/w) collagen concentration and a controlled rate of freezing of 0.92 °C/min. Morphologically, the scaffolds presented with a large amount of collagen sheets and polygonal pores with an average diameter of 62.18 µm (SD = 34.55) (). These matrices were formed by using a 0.6% (w/w) collagen concentration and a controlled freeze rate of 0.92 °C/min. An exact mimic of normal dermal architecture as depicted in is difficult to obtain and the described methodology of fabrication can at best address aspects of bioactivity.Citation12 The fabricated scaffolds demonstrated sufficient resistance against enzymatic degradation in vitro and follow on previous work by the author.Citation18,Citation19 The in vivo integrity of the matrices was confirmed through the use of an animal model. Scaffolds were implanted after surgical excision of normal skin on the dorsum of female Sprague-Dawley rats (South African Vaccine Producers) after obtaining ethical approval (AUCC approval No. H005-10). Data confirmed cellular infiltration on day 7 while scaffold integrity was retained. The cell population consisted typically of fibroblasts, lymphocytes, and a few neutrophils (). The H&E stained sections () confirm the bioactivity and the scaffold afforded interaction with cellular components. Wound healing on day 28 () presented with a superficial layer having a scar tissue appearance (rich in type III collagen fibers) and a deeper neodermis (dominated by type I collagen fibers). Deeper collagen deposition typically demonstrates a basket weave configuration with associated fibroblasts and evidence of neovascularization.
Figure 2. (A) Masson’s trichrome stained section demonstrating the intact silicone epidermal layer with a mean thickness of 1.37 ± 0.22 mm (solid arrows) and the integrated scaffold with the interface between the wound bed and scaffold (open arrows). The separation, as seen in the micrograph, was the result of shear forces during sectioning (scale bar = 1000 μm). (B) H&E stained section of the control scaffold on day 7 in vivo. The bottom left quadrant shows large numbers of lymphocytes (open arrows). The middle of the filed as well as the top left indicates fibroblast infiltration (solid arrows) (scale bar = 300 μm). (C) H&E stained section on day 28 in vivo with a superficial layer of the epidermis after healing, neo-collagenesis in the deeper lying areas, and neovascularization (solid arrows) (scale bar = 300 μm).
Any additions aimed at enhancing wound healing in vivo can only be added during the coprecipitate formation and/or after final cross-linking. These two steps serve as the points of transformation where the matrix can become a delivery system in order to promote wound healing.Citation20 Cells are typically seeded after the final fabrication and this is also true for nylon-based laminates.Citation19,Citation21 This is typically after the final coating of the knitted tri-filament mesh which is coated with an ultra-thin layer of biomedical grade silicone as reported by Wessels and Pretorius in 2013 ().Citation19 Constructs like these consist of biomaterials with mechanical properties that allows wound adhesion through the added collagen, promotes cell migration along the nylon filaments, and simultaneously serve as a physical defense against bacterial infection while permitting the flow of wound exudate.Citation10,Citation19,Citation21 The nylon tri-filament mesh allows for the covalent addition of collagen, coating with a hydrogel, or cellular seeding in vitro. TransCyte®, originally termed Dermagraft-TC, relies on a similar construct and is seeded with neonatal dermal fibroblasts.Citation21
The application of biomaterials has evolved from the development of medical devices and prostheses, to its current applications through the study of their biological interaction and intended purpose. Biomaterial selection is determined by the tissue that is targeted for replacement or augmentation. Tissue engineering strategies aimed skin replacement primarily employs biological and synthetic polymers such as collagen, nylon, siloxanes, and many others.Citation22 The safe use of these biomaterials, especially collagen, has been proven over time and their application has had a significant impact on soft tissue repair.Citation23 Alternative skin for partial-thickness burns described here has become a valuable aide to current treatment regimens and demonstrate satisfactory clinical results. However, currently available collagen-based scaffolds for full-thickness injuries fail to yield perfect results. These porous constructs allow for cell migration and nutrient and metabolite diffusion, resist biodegradation, afford appropriate biological signaling, and serve as a vehicle for extrinsic biologically active components aimed at improved healing.Citation20 The shortfall, despite these favorable attributes, could be ascribed to the fact that they are molecularly flawed as ECM mimics. Our understanding of the structure and function of the ECM and its components has advanced over the past 20 years as illustrated by the review of Eckes et al., in 2010. The interactions between cells and the ECM affects gene transcription, modifies growth factor signaling, and this in turn has an effect on ECM deposition by fibroblasts.Citation24 Work by Fisher and colleagues in 2009Citation25 and more recentlyCitation26 in 2013 demonstrated that age-related dermal collagen fragmentation negatively impacts fibroblast activity. Their findings suggest that the ECM of normal skin permits integrin-mediated fibroblast contact, which in turn regulates cell shape and normal fibroblast collagen production through mechanical interaction. This relates to their earlier work that reported de novo fibroblast collagen synthesis induced by mechanical force on the dermal ECM and cells after cross-linked hyaluronic acid has been injected to correct facial wrinkles.Citation27 Aging-related collagen fragmentation according to Fisher and colleagues has been shown to reduce fibroblast stretch. This in turn leads to an increase in oxidative stress and results in an elevation in MMP-1 (matrix metalloproteinases-1) expression. The latter further aggravates ECM breakdown and thus has further deleterious effects on fibroblast function and ECM integrity.Citation25,Citation26 The underlying principle could relate to scaffolding approaches in tissue engineering. Meticulous assembly through 3D-printing of biomaterials and scaffolds might address this and has become more feasible with advances in research.
Conclusions
Skin substitutes or engineered alternative skin remain invaluable regardless of their shortfalls. They are known to facilitate wound healing, improve patient survival, and produce satisfactory functional and cosmetic results. The use collagen, more specifically atelocollagen, as a biomaterial has led to major advances in the development of engineered alternative skin and subsequent soft tissue repair. This is largely attributed to its favorable biological and physiochemical properties. Collagen can easily be transformed, as described by the author, into porous scaffolds and combined with other biomaterials such as nylon-silicone laminates. Porous dermal regeneration scaffolds can be obtained through a sequence of steps that includes the formation of a coprecipitate, controlled freezing, lyophilization, and crosslinking. Yet, current off-the-shelf full-thickness substitutes fail to regenerate accessory skin structures, create neurovascular bundles, and deliver scarless healing. Biomaterials and tissue engineering scaffolds produced through rapid prototyping and 3D-printing will replace those developed through conventional fabrication strategies. These engineered alternative skin replacements will have the potential to yield improved results both clinically and cosmetically. This is due to the fact that perfect ECM mimics require the exact molecular composition as the healthy in vivo counterpart they aim to reproduce.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Acknowledgments
This study was funded by Southern Group of Companies, Gauteng, South Africa.
References
- MacNeil S. Progress and opportunities for tissue-engineered skin. Nature 2007; 445:874 - 80; http://dx.doi.org/10.1038/nature05664; PMID: 17314974
- Woodroof EA, Phipps RP, Greenwood JE, Hickerson W, Herndon D. The Search for an Ideal Temporary Skin Substitute: AWBAT Plus, a Combination Product Wound Dressing Medical Device. Eplasty 2010; 10:e60; PMID: 20862296
- Bello YM, Falabella AF, Eaglstein WH. Tissue-engineered skin. Current status in wound healing. Am J Clin Dermatol 2001; 2:305 - 13; http://dx.doi.org/10.2165/00128071-200102050-00005; PMID: 11721649
- Jones I, Currie L, Martin R. A guide to biological skin substitutes. Br J Plast Surg 2002; 55:185 - 93; http://dx.doi.org/10.1054/bjps.2002.3800; PMID: 12041969
- Metcalfe AD, Ferguson MW. Tissue engineering of replacement skin: the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J R Soc Interface 2007; 4:413 - 37; http://dx.doi.org/10.1098/rsif.2006.0179; PMID: 17251138
- Shahrokhi S, Arno A, Jeschke MG. The use of dermal substitutes in burn surgery: acute phase. Wound Repair Regen 2014; 22:14 - 22; http://dx.doi.org/10.1111/wrr.12119; PMID: 24393152
- Yannas IV, Burke JF. Design of an artificial skin. I. Basic design principles. J Biomed Mater Res 1980; 14:65 - 81; http://dx.doi.org/10.1002/jbm.820140108; PMID: 6987234
- Yannas IV, Burke JF, Gordon PL, Huang C, Rubenstein RH. Design of an artificial skin. II. Control of chemical composition. J Biomed Mater Res 1980; 14:107 - 32; http://dx.doi.org/10.1002/jbm.820140203; PMID: 7358747
- Woodroof EA. Biobrane: a new biosynthetic skin substitute. Care of the burn wound, 6th International Congress; 1983 29 August - 4September; Switzerland, Geneva, 1983. p105.
- Sheridan RL, Tompkins RG. Skin substitutes in burns. Burns 1999; 25:97 - 103; http://dx.doi.org/10.1016/S0305-4179(98)00176-4; PMID: 10208382
- Burd A, Ahmed K, Lam S, Ayyappan T, Huang L. Stem cell strategies in burns care. Burns 2007; 33:282 - 91; http://dx.doi.org/10.1016/j.burns.2006.08.031; PMID: 17329028
- Chan BP, Leong KW. Scaffolding in tissue engineering: general approaches and tissue-specific considerations. Eur Spine J 2008; 17:Suppl 4 467 - 79; http://dx.doi.org/10.1007/s00586-008-0745-3; PMID: 19005702
- Hodgkinson T, Bayat A. Dermal substitute-assisted healing: enhancing stem cell therapy with novel biomaterial design. Arch Dermatol Res 2011; 303:301 - 15; http://dx.doi.org/10.1007/s00403-011-1131-2; PMID: 21365208
- Burke JF, Yannas IV, Quinby WC Jr., Bondoc CC, Jung WK. Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann Surg 1981; 194:413 - 28; http://dx.doi.org/10.1097/00000658-198110000-00005; PMID: 6792993
- Yannas IV. Preparation of collagen-glycosaminoglycan copolymers for tissue regeneration. In: Morgan JR, Yarmush ML, editors. Tissue engineering methods and protocols. New Jersey: Humana Press, 1999. p3-18.
- Olde Damink LHH, Dijkstra PJ, van Luyn MJ, van Wachem PB, Nieuwenhuis P, Feijen J. Cross-linking of dermal sheep collagen using a water-soluble carbodiimide. Biomaterials 1996; 17:765 - 73; http://dx.doi.org/10.1016/0142-9612(96)81413-X; PMID: 8730960
- Hara M. Various crosslinking methods for collagens: merit and demerit of methods by radiation. J Oral Tissue Eng 2006; 3:118 - 4
- Wessels QB, Pretorius E. Enhanced stabilization of collagen-based dermal regeneration scaffolds through the combination of physical and chemical crosslinking. S Afr J Sci 2008; 104:513 - 6; http://dx.doi.org/10.1590/S0038-23532008000600030
- Wessels Q, Pretorius E. Development and ultra-structure of an ultra-thin silicone epidermis of bioengineered alternative tissue. Int Wound J 2013; http://dx.doi.org/10.1111/iwj.12126; PMID: 23834497
- Berger J, Reist M, Mayer JM, Felt O, Peppas NA, Gurny R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur J Pharm Biopharm 2004; 57:19 - 34; http://dx.doi.org/10.1016/S0939-6411(03)00161-9; PMID: 14729078
- Purdue GF, Hunt JL, Still JM Jr., Law EJ, Herndon DN, Goldfarb IW, Schiller WR, Hansbrough JF, Hickerson WL, Himel HN, et al. A multicenter clinical trial of a biosynthetic skin replacement, Dermagraft-TC, compared with cryopreserved human cadaver skin for temporary coverage of excised burn wounds. J Burn Care Rehabil 1997; 18:52 - 7; http://dx.doi.org/10.1097/00004630-199701000-00009; PMID: 9063788
- Hench LL, Jones JR. Biomaterials, artificial organs and tissue engineering. Cambridge: Woodhead publishing limited, 2005.
- Pachence JM. Collagen-based devices for soft tissue repair. J Biomed Mater Res 1996; 33:35 - 40; http://dx.doi.org/10.1002/(SICI)1097-4636(199621)33:1<35::AID-JBM6>3.0.CO;2-N; PMID: 8734072
- Eckes B, Nischt R, Krieg T. Cell-matrix interactions in dermal repair and scarring. Fibrogenesis Tissue Repair 2010; 3:4; http://dx.doi.org/10.1186/1755-1536-3-4; PMID: 20222960
- Fisher GJ, Quan T, Purohit T, Shao Y, Cho MK, He T, Varani J, Kang S, Voorhees JJ. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am J Pathol 2009; 174:101 - 14; http://dx.doi.org/10.2353/ajpath.2009.080599; PMID: 19116368
- Quan T, Little E, Quan H, Qin Z, Voorhees JJ, Fisher GJ. Elevated matrix metalloproteinases and collagen fragmentation in photodamaged human skin: impact of altered extracellular matrix microenvironment on dermal fibroblast function. J Invest Dermatol 2013; 133:1362 - 6; http://dx.doi.org/10.1038/jid.2012.509; PMID: 23466932
- Wang F, Garza LA, Kang S, Varani J, Orringer JS, Fisher GJ, Voorhees JJ. In vivo stimulation of de novo collagen production caused by cross-linked hyaluronic acid dermal filler injections in photodamaged human skin. Arch Dermatol 2007; 143:155 - 63; http://dx.doi.org/10.1001/archderm.143.2.155; PMID: 17309996
