Abstract
Prosthetic joints and other orthopedic implants have improved quality of life for patients world-wide and the use of such devices is increasing. However, while infection rates subsequent to associated surgery are relatively low (<3%), the consequences of incidence are considerable, encompassing morbidity (including amputation) and mortality in addition to significant social and economic costs. Emphasis, therefore, has been placed on mitigating microbial risk, with clinical microbiologists and surgeons utilizing rapidly evolving molecular laboratory techniques in detection and diagnosis of infection, which still occurs despite sophisticated patient management. Multidisciplinary approaches are regularly adopted to achieve this. In this commentary, we describe an unusual case of Actinomyces infection in total hip arthroplasty and, in that context, describe the perspectives of the clinical microbiology and surgical teams and how they contrasted. More specifically, this case demonstrates an ad hoc approach to structured eradication of biofilms and intracellular bacteria related to biomaterials, as reflected in early usage of linezolid. This is a complex topic and, as described in this case, such accelerated treatment can be effective. This commentary focuses on the merits of such inadvisable use of potent antimicrobials amid the risk of diminishing valuable antimicrobial efficacy, albeit resulting in desirable patient outcomes.
Introduction
North America has experienced significant declines in mortality secondary to infectious diseases.Citation1 In the European Union, incidence of severe sepsis, associated with multiorgan failure, is currently estimated as 90.4 cases per 100 000 population.Citation2 Not least, in bringing about improvements, have been the adoption of enhanced infection control practices, efficacious antimicrobial therapies, advanced molecular-based laboratory methodologies and sepsis management protocols.Citation3 Indeed, direct links have been demonstrated between time required for pathogen identification and infectious illness outcomes.Citation4 In response, use of molecular-based laboratory technologies has become relatively common, leading to more rapid microbial identification, susceptibility testing, reduction in empiric antimicrobial use, faster application of targeted therapies, and, overall, enhanced patient care.Citation5
Despite these advances, surgical practice can require decision-making with respect to treatment of nosocomial or procedure-related infections, often in the absence of laboratory data regarding the nature of causative organisms, whether those infections are monomicrobial or polymicrobial, and their relative sensitivities to available bacteriostatic or bacteriocidal agents. There is, therefore, an imperative that clinical and molecular microbiologists determine accurate etiological diagnoses enabling selection of the most appropriate antimicrobial treatments. However, while laboratory analysis is underway, surgical teams may decide to engage in broad-spectrum empiric treatments, using parenteral or oral therapy, as well as consideration of further surgical management.
In the case of orthopedic surgery, specifically, the use of prosthetic joints and other implants has been associated with relatively low levels of infection (<3%).Citation6 Focusing more keenly on total hip arthroplasty, or hip replacement, reports of infection classify occurrences based on the timing of incidence: “early” within one month of procedure, “delayed” within a year, and “late” at any time after that.Citation7,Citation8 Such infections are considered a serious complication,Citation9 with “early” and “delayed” infection typically due to perioperative bacterial contamination, whereas “late” incidence is understood to be predominantly blood-borne.Citation7,Citation10
The Case
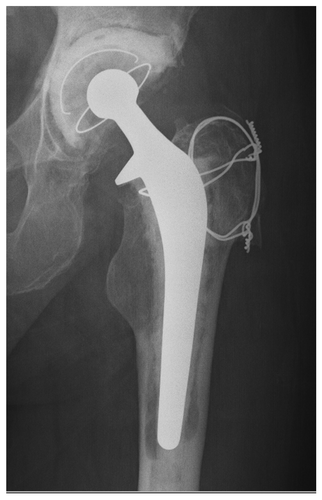
Prosthetic joint infections typically result from monomicrobial contamination by Staphylococcus aureus or S. epidermis, with much fewer cases associated with any other species.Citation11-Citation15 In this case, a 71 year old man had presented 9 years following hip arthroplasty with hip pain and elevated inflammatory markers (erythrocyte sedimentation rate of 71 mm/h and c-reactive protein of 65 mg/l).Citation7 Imaging techniques indicated that there was evidence of osteolysis, subsequent biopsies of adjacent tissue confirmed presence of Actinomyces israelii. Treatment involved removal of the prosthesis, insertion of vancomycin-loaded bone cement spacer, intravenous antimicrobials, and eventual re-implantation with a new prosthesis. There has been no recurrence of infection to date.
The Microbiologist Perspective
Actinomyces israelii is a filamentous Gram-positive anaerobic bacterium, is considered only opportunistically pathogenic,Citation16 and is frequently isolated from the gastrointestinal tract, bronchi, oral cavities, and female genital tract.Citation14,Citation17 Pathogenesis most commonly involves dental caries or gingival disease, with infections of the lung or abdomen being the next most common. A. israelii infection in hip replacement is extremely rare with only 3 previous reported cases in the literature, associated with contamination at the time of surgery,Citation13 dental work without antibiotic prophylaxis,Citation14 and intravenous drug abuse.Citation17 In the present case, infection occurred with Type 2 diabetes mellitus being the dominant risk factor.
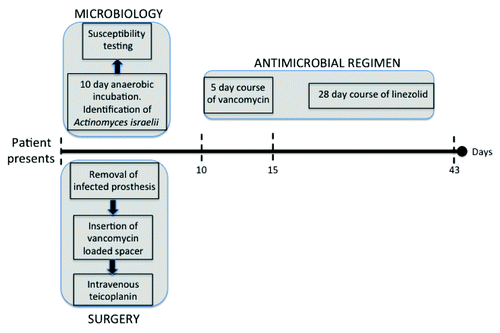
For the reasons outlined above, Actinomyces infection was not initially suspected in this case. Indeed, aspirate from the painful hip joint was devoid of microbes () and it was only following culture of biopsies from periprosthetic tissue that A. israelii was detected. The microbiological process () involved 10 days anaerobic incubation on blood agar and use of biochemical kits (bioMérieux®API®). Laboratory assays demonstrated susceptibility to penicillin, teicoplanin, vancomycin, ciprofloxacin, and linezolid. These results corresponded broadly with expert recommendations for antimicrobial therapyCitation18,Citation19 including prolonged high doses of parenteral penicillin, followed by oral penicillin and/or amoxicillin or tetracyclin, erythromycin, doxycycline, or clindamycin if penicillin is not an option.Citation20
The Surgeon Perspective
An empirical antimicrobial approach was adopted (). A vancomycin-loaded cement spacer was put in place and intravenous teicoplanin administered until the infectious agent was identified and susceptibility to vancomycin was confirmed by the clinical microbiology team, at which point teicoplanin was discontinued. The subsequent therapeutic approach deviated from recommended anti-actinomycosis protocols,Citation20 through relatively long-term (28 days) use of linezolid, due to the following factors:
Requirement for eradication of infection before any re-implantation could occur
Recognition that biofilm formation, initially associated with the pathogenesis of catheter-related infection but now considered a key aspect of many biomaterial-related microbes, may be involved.Citation21 This was potentially indicated by the lack of recoverable bacteria from the aspirated synovial fluid.
Linezolid has demonstrated efficacy against most Gram-positive pathogens, including multidrug-resistant staphylococci.Citation22,Citation23
Linezolid has been shown to accumulate rapidly in bone, with reported efficacy in a broad range of orthopedic infections.Citation24
Discussion
Among the primary roles of the clinical microbiologist are guidance and support of surgical teams, and selection of appropriate diagnostic investigations and antimicrobials, as warranted.Citation5 In this case, the recommended antibiotic treatment profile, based on susceptibility testing, included teicoplanin and vancomycin. However, the imperative for the surgical team was eradication of Actinomyces associated with bone prosthesis. In that context, linezolid was a suitable antimicrobial due to its proven ability to achieve high concentrations in bone,Citation24 and so the patient was administered a four week oral course (2 × 600 mg day−1).
From a clinical microbiologist perspective, this course of treatment would be undesirable as linezolid is generally reserved for treatment of multidrug-resistant microbes. Worryingly, although resistance to linezolid is difficult to generate in vitro,Citation23 emergence of cfr-related in vivo resistance has been reportedCitation25-Citation27 in addition to only bacteriostatic activity against staphylococci and Enterococcus spp.Citation28 Indeed, pharmacodynamic studies provide evidence of low AUC24/MIC related to high numbers of therapeutic failure,Citation28 including orthopedic applications.Citation29,Citation30 Of even greater concern to the clinical microbiologist, however, are reports of adverse events associated with relatively long-term use of linezolid,Citation31,Citation32 analogous to the four week regimen in this case (although linezolid is approved for treatment of that duration).
The surgeon-led patient-centered care commented on here focused on efficacy of treatment appropriate to an elderly man. Since approximately 1996, management of similar cases has employed both two-stage surgery (i.e., removal of infected prostheses—sometimes use of antibiotic-loaded spacers—eradication of causative pathogens followed by replacement of devices) and intracellular antimicrobials to avoid relapse due to potential harboring of bacteria within periprosthetic fibroblasts.Citation33 That was the approach adopted in this case, oral linezolid facilitating outpatient-based treatment and, importantly for the elderly patient, markedly reduced discomfort for him and his family.
In conclusion, advances in molecular technologies for rapid species identification and susceptibility testing are mitigating the protracted incubation times associated with conventional microbiology, facilitating quicker diagnosis and reduction in exposure of patients to empiric therapy in favor of targeted antimicrobial use. In the specific case described here, the use of linezolid proved successful, with no adverse events evident. However, it is probable that double-blind, randomized trials of linezolid in orthopedic settings are necessary to clarify its efficacy and, therefore, suitability for use.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
References
- Armstrong GL, Conn LA, Pinner RW. Trends in infectious disease mortality in the United States during the 20th century. JAMA 1999; 281:61 - 6; http://dx.doi.org/10.1001/jama.281.1.61; PMID: 9892452
- Daniels R. Surviving the first hours in sepsis: getting the basics right (an intensivist’s perspective). J Antimicrob Chemother 2011; 66:Suppl 2 ii11 - 23; http://dx.doi.org/10.1093/jac/dkq515; PMID: 21398303
- Phua J, Ho BC, Tee A, Chan KP, Johan A, Loo S, So CR, Chia N, Tan AY, Tham HM, et al. The impact of clinical protocols in the management of severe sepsis: a prospective cohort study. Anaesth Intensive Care 2012; 40:663 - 74; PMID: 22813495
- Yang S, Rothman RE. PCR-based diagnostics for infectious diseases: uses, limitations, and future applications in acute-care settings. Lancet Infect Dis 2004; 4:337 - 48; http://dx.doi.org/10.1016/S1473-3099(04)01044-8; PMID: 15172342
- O’Connor C, Fitzgibbon M, Powell J, Barron D, O’ Mahony J, Power L, O’Connell NH, Dunne C. A commentary on the role of molecular technology and automation in clinical diagnostics. Bioengineered 2014; 5; In press PMID: 24658184
- Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med 2004; 350:1422 - 9; http://dx.doi.org/10.1056/NEJMra035415; PMID: 15070792
- Wu F, Marriage NA, Ismaeel A, Masterson E. Infection of a total hip arthroplasty with actinomyces israelii: Report of a case. N Am J Med Sci 2011; 3:247 - 8; http://dx.doi.org/10.4297/najms.2011.3247; PMID: 22558603
- Zimmerli W, Ochsner PE. Management of infection associated with prosthetic joints. Infection 2003; 31:99 - 108; http://dx.doi.org/10.1007/s15010-002-3079-9; PMID: 12682815
- Nho SJ, Kymes SM, Callaghan JJ, Felson DT. The burden of hip osteoarthritis in the United States: epidemiologic and economic considerations. J Am Acad Orthop Surg 2013; 21:Suppl 1 S1 - 6; http://dx.doi.org/10.5435/JAAOS-21-07-S1; PMID: 23818185
- Cordero-Ampuero J, de Dios M. What are the risk factors for infection in hemiarthroplasties and total hip arthroplasties?. Clin Orthop Relat Res 2010; 468:3268 - 77; http://dx.doi.org/10.1007/s11999-010-1411-8; PMID: 20544319
- Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR, Infectious Diseases Society of America. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2013; 56:e1 - 25; http://dx.doi.org/10.1093/cid/cis803; PMID: 23223583
- Sendi P, Christensson B, Uçkay I, Trampuz A, Achermann Y, Boggian K, Svensson D, Widerström M, Zimmerli W, GBS PJI study group. Group B streptococcus in prosthetic hip and knee joint-associated infections. J Hosp Infect 2011; 79:64 - 9; http://dx.doi.org/10.1016/j.jhin.2011.04.022; PMID: 21764170
- Petrini B, Welin-Berger T. Late infection with Actinomyces israelii after total hip replacement. Scand J Infect Dis 1978; 10:313 - 4; PMID: 725541
- Strazzeri JC, Anzel S. Infected total hip arthroplasty due to Actinomyces israelii after dental extraction. A case report. Clin Orthop Relat Res 1986; 128 - 31; PMID: 3757351
- Esteban J, Cordero-Ampuero J. Treatment of prosthetic osteoarticular infections. Expert Opin Pharmacother 2011; 12:899 - 912; http://dx.doi.org/10.1517/14656566.2011.543676; PMID: 21405943
- Alekh K, Dazley J, Sison R, Slim J, Boghossian J.. A rare case of Actinomyces israelii bacteraemia. Journal of medical microbiology case reports 2014; 1:1 - 3
- Zaman R, Abbas M, Burd E. Late prosthetic hip joint infection with Actinomyces israelii in an intravenous drug user: case report and literature review. J Clin Microbiol 2002; 40:4391 - 2; http://dx.doi.org/10.1128/JCM.40.11.4391-4392.2002; PMID: 12409439
- Barberán J, Aguilar L, Carroquino G, Giménez MJ, Sánchez B, Martínez D, Prieto J. Conservative treatment of staphylococcal prosthetic joint infections in elderly patients. Am J Med 2006; 119:e7 - 10; http://dx.doi.org/10.1016/j.amjmed.2006.03.036; PMID: 17071171
- Rissing JP. Antimicrobial therapy for chronic osteomyelitis in adults: role of the quinolones. Clin Infect Dis 1997; 25:1327 - 33; http://dx.doi.org/10.1086/516150; PMID: 9431371
- Russo T. Agents of actinomycosis. In: Mandell G, Bennett J, Dolin R, eds. Principles and practice of infectious diseases, 6th Ed. New York: Churchill Livingstone, 2005:2924-34.
- Del Pozo JL, Patel R. Clinical practice. Infection associated with prosthetic joints. N Engl J Med 2009; 361:787 - 94; http://dx.doi.org/10.1056/NEJMcp0905029; PMID: 19692690
- Moellering RC. Linezolid: the first oxazolidinone antimicrobial. Ann Intern Med 2003; 138:135 - 42; http://dx.doi.org/10.7326/0003-4819-138-2-200301210-00015; PMID: 12529096
- Livermore DM. Linezolid in vitro: mechanism and antibacterial spectrum. J Antimicrob Chemother 2003; 51:Suppl 2 ii9 - 16; http://dx.doi.org/10.1093/jac/dkg249; PMID: 12730138
- Venugopalan V, Martin CA. Selecting anti-infective agents for the treatment of bone infections: new anti-infective agents and chronic suppressive therapy. Orthopedics 2007; 30:832 - 4; PMID: 17990408
- Morales G, Picazo JJ, Baos E, Candel FJ, Arribi A, Peláez B, Andrade R, de la Torre MA, Fereres J, Sánchez-García M. Resistance to linezolid is mediated by the cfr gene in the first report of an outbreak of linezolid-resistant Staphylococcus aureus. Clin Infect Dis 2010; 50:821 - 5; http://dx.doi.org/10.1086/650574; PMID: 20144045
- Cui L, Wang Y, Li Y, He T, Schwarz S, Ding Y, Shen J, Lv Y. Cfr-mediated linezolid-resistance among methicillin-resistant coagulase-negative staphylococci from infections of humans. PLoS One 2013; 8:e57096; http://dx.doi.org/10.1371/journal.pone.0057096; PMID: 23437319
- Gu B, Kelesidis T, Tsiodras S, Hindler J, Humphries RM. The emerging problem of linezolid-resistant Staphylococcus. J Antimicrob Chemother 2013; 68:4 - 11; http://dx.doi.org/10.1093/jac/dks354; PMID: 22949625
- MacGowan AP. Pharmacokinetic and pharmacodynamic profile of linezolid in healthy volunteers and patients with Gram-positive infections. J Antimicrob Chemother 2003; 51:Suppl 2 ii17 - 25; http://dx.doi.org/10.1093/jac/dkg248; PMID: 12730139
- Razonable RR, Osmon DR, Steckelberg JM. Linezolid therapy for orthopedic infections. Mayo Clin Proc 2004; 79:1137 - 44; http://dx.doi.org/10.1016/S0025-6196(11)62596-2; PMID: 15357035
- Rao N, Ziran BH, Hall RA, Santa ER. Successful treatment of chronic bone and joint infections with oral linezolid. Clin Orthop Relat Res 2004; 67 - 71; http://dx.doi.org/10.1097/01.blo.0000144860.11193.5e; PMID: 15552139
- Harwood PJ, Talbot C, Dimoutsos M, Sunderland G, Shaw D, Wilcox MH, Giannoudis PV. Early experience with linezolid for infections in orthopaedics. Injury 2006; 37:818 - 26; http://dx.doi.org/10.1016/j.injury.2006.02.007; PMID: 16620816
- Papadopoulos A, Plachouras D, Giannitsioti E, Poulakou G, Giamarellou H, Kanellakopoulou K. Efficacy and tolerability of linezolid in chronic osteomyelitis and prosthetic joint infections: a case-control study. J Chemother 2009; 21:165 - 9; http://dx.doi.org/10.1179/joc.2009.21.2.165; PMID: 19423469
- Sendi P, Rohrbach M, Graber P, Frei R, Ochsner PE, Zimmerli W. Staphylococcus aureus small colony variants in prosthetic joint infection. Clin Infect Dis 2006; 43:961 - 7; http://dx.doi.org/10.1086/507633; PMID: 16983605