Abstract
Gluconacetobacter diazotrophicus is a gram-negative and endophytic nitrogen-fixing bacterium that has several beneficial effects in host plants; thus, utilization of this bacterium as a biofertilizer in agriculture may be possible. G. diazotrophicus synthesizes levan, a D-fructofuranosyl polymer with β-(2→6) linkages, as an exopolysaccharide and the synthesized levan improves the stress tolerance of the bacterium. In this study, we found that phosphate enhances levan production by G. diazotrophicus Pal5, a wild type strain that showed a stronger mucous phenotype on solid medium containing 28 mM phosphate than on solid medium containing 7 mM phosphate. A G. diazotrophicus Pal5 levansucrase disruptant showed only a weak mucous phenotype regardless of the phosphate concentration, indicating that the mucous phenotype observed on 28 mM phosphate medium was caused by levan. To our knowledge, this is the first report of the effect of a high concentration of phosphate on exopolysaccharide production.
Introduction
Due to the increasing cost of chemical nitrogenous fertilizers and concerns about contamination of soil and water, there is a need to reduce usage of chemical fertilizer.Citation1 Plant-associated microorganisms containing nitrogenases have attracted attention as alternative biofertilizers,Citation2 since nitrogenase catalyzes a reaction referred to as biological nitrogen fixation, in which atmospheric nitrogen is converted to ammonia.
Plant-associated nitrogen-fixing microorganisms reside in the internal parts of plant and the rhizosphere, providing host plants with nitrogenous compounds,Citation3 while the host plants supply the microorganisms with nutrients such as carbon sources, organic acids, and amino acids. One such plant-associated microorganism, Gluconacetobacter diazotrophicus, is a gram-negative, obligate aerobic, and endophytic nitrogen-fixing bacterium that was originally isolated from sugarcane.Citation4 This bacterium has also been isolated from natural Ipomoea batatas (sweet potato),Citation5 Coffea arabica L. (coffee),Citation6 Pennisetum purpureum (cameroon grass),Citation7 and Ananas comosus (pineapple).Citation8 As well as serving as a nitrogen source for the host plant, G. diazotrophicus produces phytohormones such as indole acetic acid and gibberellic acid,Citation9-Citation11 and antimicrobial compounds against phytopathogenic Xanthomonas albilineans.Citation12 G. diazotrophicus can also solubilize insoluble metals in vitro.Citation13,Citation14 Thus, utilization of G. diazotrophicus as a biofertilizer in agriculture may allow reduced use of chemical fertilizers.
G. diazotrophicus cells mainly inhabit the host plant, and the survival rate is very low when the bacterium is inoculated artificially in soil.Citation15 Thus, use of G. diazotrophicus as a biofertilizer requires a study of its physiological properties, including how this bacterium responds to extracellular compounds in the environment. In this study, we unexpectedly found mucous growth of G. diazotrophicus Pal5 on a solid medium with a high concentration of phosphate. We show that the mucous trait is caused by production of levan, a linear fructose polymer that is enhanced by a high concentration of phosphate.
Results
A high mucous phenotype of G. diazotrophicus Pal5 at a high phosphate concentration
In growth experiments on solid media such as LGI-P, C2-NaCl, Dygs, Y and P, and Y and P-NaCl, the G. diazotrophicus Pal5 strain showed a stronger mucous phenotype on solid Y and P and Y and P-NaCl medium than on solid LGI-P medium (data not shown). LGI-PCitation16 is the medium used for G. diazotrophicus (), Y and P medium has been used for E. coli carrying nif clusters of Klebsiella pneumonia,Citation17 and Y and P-NaCl medium is Y and P medium that lacks NaCl and thiamine ().
Table 1. Composition of LGI-P, Y&P-NaCl, high P, and low P media
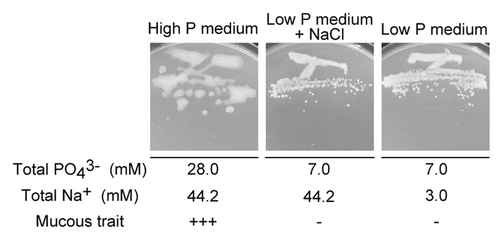
To identify the ingredient(s) in the medium that led to the highly mucous phenotype, we initially showed that each ingredient specific to LGI-P medium (FeCl3, CaCl2, biotin, and pyridoxal; ) had no effect on the highly mucous trait of G. diazotrophicus Pal5, excluding the possibility that these ingredients caused the highly mucous phenotype. We then focused on the phosphate concentrations in the LGI-P and Y and P-NaCl media, which are 6 mM and 50 mM, respectively (). To check the effect of this concentration, growth of the Pal5 strain was examined on solid low P medium (), in which the 6.25 g/L Na2HPO4 in Y and P-NaCl medium was reduced to 0.20 g/L (final phosphate, 7.0 mM). The mucous trait of the cells was significantly reduced on solid low P medium (), suggesting that a high concentration of Na2HPO4 caused the highly mucous phenotype of the Pal5 strain.
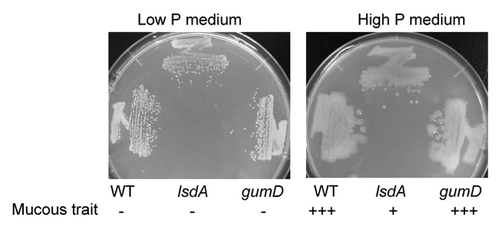
Figure 1.G. diazotrophicus Pal5 WT, gumD mutant, and lsdA mutant strains grown on high or low P solid medium. Plus (+++) indicates the strongest mucous trait, plus (+) is moderate, and minus (-) is the weakest. Cells were grown for 1 wk at 30 °C.
The Pal5 strain still exhibited a highly mucous phenotype on solid high P medium (), in which the 6.25 g/L Na2HPO4 in Y and P-NaCl medium was reduced to 3.13 g/L (final phosphate, 28 mM). The Pal5 strain also had a mucous trait regardless of use of a sodium or potassium salt, since the strain showed a highly mucous phenotype on medium in which 6.25 g/L Na2HPO4 in Y and P-NaCl medium was replaced by 6.25 g/L K2HPO4 (data not shown). The highly mucous phenotype of the Pal5 strain also occurred on solid high P medium with adjustment of its normal pH 7.5 to 6.3, whereas the less mucous phenotype occurred on solid low P medium with adjustment of its normal pH 6.3 to 7.5 (data not shown). These findings indicate that the pH of the medium was not involved in the highly mucous phenotype. To examine the effect of a high concentration of Na+, G. diazotrophicus Pal5 was grown on a medium containing 7 mM phosphate and 45 mM Na+ (; ). No mucous phenotype occurred with this medium, indicating that a high concentration of Na+ was not associated with the mucous phenotype. Collectively, these data show that a high P concentration (>28 mM) enhances the highly mucous phenotype of G. diazotrophicus Pal5.
Enhanced levan production by G. diazotrophicus Pal5 at a high phosphate concentration
To identify the components in the mucous material, a G. diazotrophicus gumD disruptant (MK4004) strain was constructed. The gumD gene codes for a protein that is probably responsible for the first step in extracellular polysaccharide (EPS) production. The amount of EPS produced by the gumD disruptant of the Pal5 strain in liquid LGI-based medium with 20 g/L sucrose is reduced by approximately 50% compared with that of wild type (WT).Citation18 However, the gumD disruptant still formed highly mucous colonies on solid high P medium, similarly to the WT strain ().
A G. diazotrophicus lsdA disruptant was grown in the same way. The lsdA gene codes for levansucrase, an extracellular fructosyltransferase that catalyzes synthesis of levan from sucrose. Levan is a linear fructose polymer with β-(2→6) links and more than 100 fructosyl residues. The mucous trait of the lsdA disruptant strain was substantially lower than that of the WT strain on solid high P medium (), suggesting that the mucous material is levan.
To confirm that the mucous material was levan, mucous colonies of Pal5 cells on solid high P medium and less mucous colonies on solid low P medium were hydrolyzed and analyzed by HPLC. Authentic samples of hydrolyzed levan, sucrose, and fructose all gave a levan peak at a retention time of 15.3 min (Fig. S1; ). The levan peak was higher in hydrolyzed mucous colonies from high P medium than from hydrolyzed less mucous colonies from low P medium (; Fig. S1). Moreover, thin layer chromatography (TLC) analysis that specifically detects levan, fructose, and fructosyl derivatives demonstrated that hydrolyzed mucous colonies from high P medium contain higher amounts of fructose and fructosyl derivatives than hydrolyzed less mucous colonies from low P medium (Fig. S2). Hydrolyzed less mucous colonies of lsdA disruptant cells on solid high P medium showed a much smaller levan peak than those of WT or gumD disruptant cells on solid high P medium on HPLC analysis. These data confirm that the mucous material is levan and that a high concentration of phosphate (>28 mM) enhances production of levan by Pal5 cells on a solid medium.
Figure 3. HPLC analysis of mucous materials. Authentic levan and mucous material collected from colonies of WT, gumD, and lsdA cells grown for 1 wk at 30 °C on the indicated solid media were hydrolyzed and analyzed. The vertical axis of the chromatograph shows the relative peak level. The retention time (min) is shown on the horizontal axis. Arrows show the levan peak (15.3 min).
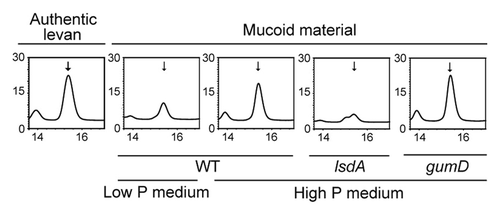
To examine whether a high concentration of phosphate (>28 mM) enhances production of levan by Pal5 cells in a liquid medium, Pal5 cells were grown aerobically in liquid high and low P media, and dried EPS (including levan) in the supernatant was weighed, hydrolyzed, and analyzed. The amounts of dried EPS from the two cultures were about the same: 35 ± 2 mg (n = 4; from 25 ml supernatant of high P medium) and 33 ± 6 mg (n = 4; from 25 ml supernatant of low P medium), and gave levan peaks of the same height (data not shown). This indicates that enhanced production of levan by phosphate is a phenotype specific to a solid medium, and does not occur in a liquid medium.
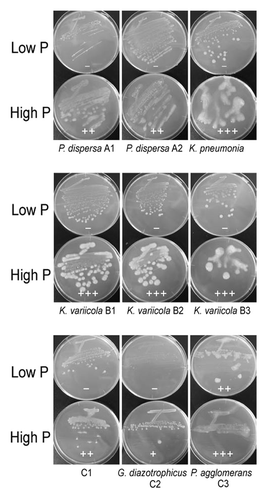
Sugarcane juices were prepared from sugarcanes a1, a2, a3, b1, b2, b3, c1, c2, and c3 (). Bacteria were isolated from each sugarcane juice and were tentatively identified as Pantoea dispersa A1, Pantoea dispersa A2, Klebsiella pneumonia A3, Klebsiella variicola B1, Klebsiella variicola B2, Klebsiella variicola B3, Gluconacetobacter diazotrophicus C2, and Pantoea agglomerans C3, respectively, based on the rDNA sequences (Fig. S3). The phosphate concentrations in the sugarcane juices were 2.8–10.8 mM (). All isolated bacteria showed a stronger mucous phenotype on solid high P medium than on solid low P medium (). However, these highly mucous phenotypes were not attributable to levan alone, since levan peaks were not observed in A3 cells and were very weak in other cells (Fig. S4).
Table 4. Concentration of phosphate in sugarcane juice
Tolerance to hydrogen peroxide
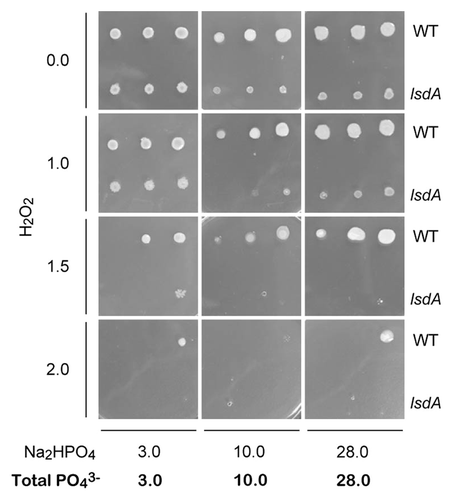
To examine whether the tolerance of G. diazotrophicus Pal5 to reactive oxygen species (ROS) is improved by levan, we cultivated G. diazotrophicus Pal5 WT and its lsdA disruptant on solid media containing hydrogen peroxide (H2O2) and 0, 3, 10, and 28 mM phosphate (), since the phosphate concentrations of sugarcane juices were determined to be 2.8 to 10.8 mM (). The lsdA disruptant exhibited lesser growth and lesser mucous growth on solid media with 28 mM phosphate and “physiological” phosphate concentrations (3 and 10 mM) in the absence and presence of hydrogen peroxide. Notably, the lsdA disruptant showed particularly marked reduction in growth in the presence of hydrogen peroxide and the phosphate concentration had no effect on tolerance (). HPLC analysis confirmed that G. diazotrophicus Pal5 synthesizes levan in the presence of 3, 10, and 28 mM phosphate (Fig. S1B). Collectively, these data suggest that levan is important for ROS resistance of G. diazotrophicus Pal5 and that a high concentration (28 mM) of phosphate has no effect on ROS resistance.
Discussion
Utilization of G. diazotrophicus strains as biofertilizer in agriculture requires an improved understanding of their physiological properties, including how this bacterium responds to extracellular compounds in the environment. Here, we found that G. diazotrophicus forms highly mucous colonies and produces a higher amount of levan on a solid medium with a high concentration of phosphate (>28 mM).
Production of levan by G. diazotrophicus strains may occur due to the ability of this bacterium to assimilate sucrose.Citation19-Citation21 In particular, Arrieta et al. found that the G. diazotrophicus SRT4 strain forms mucous colonies on solid sucrose-containing LGIE medium,Citation20 although the amount of mucus was not described. LGIE medium is a LGI-based medium that contains (in g/L) tryptone, 1; yeast extract, 0.2; sucrose, 50; and glycerol, 10; plus LGI saltsCitation4 (LGI-P, )Citation16 including 6 mM phosphate, but not glucose, ammonium sulfate, biotin, and pyridoxal. Previous studies of G. diazotrophicusCitation4,Citation19,Citation22 have used this LGI-based medium. Thus, the effects of a high phosphate concentration on production of levan have not been examined, since the LGI-based medium contains only 6 mM phosphate.
Disruption of levansucrase genes in other several bacteria impairs their behavior in association with plants or animals. A disruption of the Paenibacillus polymyxa levansucrase gene impaired its ability to aggregate soil in the wheat rhizosphere.Citation23 Levansucrase mutants of fireblight pathogen Erwinia amylovora caused retarded development of necrotic symptoms on inoculated pear seedlings.Citation24 The extracellular fructosyltransferase-deficient strain of Streptococcus mutans was less pathogenic compared with the wild-type strain.Citation25 In the case of G. diazotrophicus, levan improves stress tolerances, desiccation, osmotic pressure, and NaCl stress of this bacterium.Citation22 ROS resistance is also important for G. diazotrophicus Pal5 in colonization of plants because plants generate superoxide against pathogens as a defense mechanism.Citation26 Thus, a G. diazotrophicus disruptant strain in which ROS-detoxifying genes are destroyed is unable to colonize plant roots efficiently.Citation27 Use of an lsdA disruptant in this study suggested that levan itself is significant for tolerance of hydrogen peroxide, and G. diazotrophicus Pal5 was found to synthesize levan on a solid medium containing “physiological” concentrations (3 and 10 mM) of phosphate. This suggests that levan may facilitate this bacterium to colonize and reside in plants by improving tolerance to desiccation, osmotic pressure, and NaCl stress,Citation22 and increasing tolerance to ROS, although the physiological role of the highly mucous phenotype of Pal5 caused by a high concentration of phosphate remains unclear. With regard to the structures of the EPS produced by G. diazotrophicus, other than levan, little has been elucidated. It has been recently reported that G. diazotrophicus Pal5 produced the EPS that has 4-O-substituted units of β-glucose, 3-O-substituted units of β-galactose and 2-O-substituted units of α-mannose in the liquid medium containing mannitol.Citation28
Finally, our finding that synthesis of levan by G. diazotrophicus Pal5 is increased on a solid medium containing a high concentration of phosphate may be important for industrial production of levan. Levan is currently synthesized on an industrial scale using bacterium such as Bacillus species.Citation29 HypocholesterolemicCitation30 and cosmeceuticalCitation31 effects of levan have been reported and application of levan is likely in a variety of industrial fields, including food, cosmetics, and medicine.
Materials and Methods
Bacterial strains and growth conditions
G. diazotrophicus Pal5 ATCC49037 (American Type Culture Collection) was grown at 30 °C in C2-NaCl, Dygs,Citation32 LGI-P,Citation16 Y and P-NaCl, or high and low P media. C2-NaCl medium is C2 mediumCitation33 that lacks NaCl and contains (in g/L) tryptone, 10; glucose, 15; yeast extract, 5; pH 6.5. Y and P-NaCl medium is Y and P mediumCitation17 that lacks NaCl and thiamine. The compositions of Y and P-NaCl, LGI-P, high P, and low P media are shown in . Solid medium was made by adding agar at 15 g/L. Escherichia coli DH5α was used as the cloning host and was grown at 37 °C in LB medium, which contains (in g/L) tryptone, 10; yeast extract, 5; NaCl, 10; pH 7.2.
Construction of plasmids and recombinant G. diazotrophicus Pal5 strains
Primers and plasmids are shown in and . pKTY320-kan::gumD was constructed as follows. gumD (1521 nt) was amplified by PCR with primers 1 and 2, using G. diazotrophicus Pal5 genomic DNA as a template. The PCR product was inserted into HincII-treated pUC119, yielding pUC119-gumD. Using this plasmid as a template, inverse PCR was conducted with primers 3 and 4. Kmr fragment, obtained by SalI digestion of pUC4K, was inserted by in-fusion (Clontech) into the amplified DNA fragment, resulting in pUC119-kan::gumD. Using this plasmid as a template, the Kmr-inserted gumD was amplified by PCR with primers 5 and 6. The amplified fragment was inserted by in-fusion into HincII-treated pKTY320, giving pKTY320-kan::gumD.
Table 2. Primers used in the study a
Table 3. Plasmids used in the study
pKTY320-kan::lsdA was similarly constructed. A DNA fragment containing a part of lsdA (1460 nt: from nt 247 to 1706 in a 1755 nt full-length lsdA) was amplified by PCR with primers 7 and 8, using the Pal5 genomic DNA as a template. The PCR product was inserted into HincII-treated pUC118, yielding pUC118-lsdA-2BamHI. Using this plasmid as a template, this DNA fragment was amplified by PCR with primers 9 and 10. The PCR product was digested with XmaI/HindIII and ligated into XmaI/HindIII-treated pUC118, resulting in pUC118-lsdA-1BamHI. The Kmr fragment obtained by BamHI digestion of pUC4K was inserted into the BamHI site of pUC118-lsdA-1BamHI to give pUC118::kan-lsdA. Using this plasmid as a template, the Kmr-inserted lsdA was amplified by PCR with primers 11 and 12. The amplified fragment was inserted by in-fusion into HincII-treated pKTY320, yielding pKTY320-kan::lsdA.
G. diazotrophicus Pal5 mutant strains (MK4004, gumD::Kmr in Pal5; MK4384, lsdA::Kmr in Pal5) were made by insertional mutagenesis.Citation33 pKTY320-kan::gumD or pKTY320-kan::lsdA was introduced into the G. diazotrophicus Pal5 strain by electroporation. The applied pulse conditions were 10.0 kV/cm and 5 msec using Bio-Rad Gene Pulser Xcell (Bio-Rad). Competent cells (100 µl) were mixed with 100 ng of plasmid DNA. Transformants were selected on solid Dygs medium containing kanamycin (200 µg/ml).
HPLC and TLC analyses of G. diazotrophicus Pal5 cells
G. diazotrophicus Pal5 cells for HPLC analysis were grown on membranes as follows. Pal5 cells were grown in C2-NaCl liquid medium aerobically at 30 °C overnight and collected by centrifugation at 10 000 × g for 2 min. The cells were washed 3 times with 0.9% (w/v) NaCl and diluted to OD600 of 1.0. The cells in 50 µl of this suspension were put on a membrane (diameter 13 mm, pore size 0.22 µm) that was placed on solid medium and incubated at 30 °C for 1 wk. Colonies of the cells together with mucous materials that grew on the membrane were collected with a sterilized spatula into an Eppendorf tube and weighed.
For HPLC analysis, collected and weighed cells were suspended in 4% (w/v) H2SO4 to reach 14% (w/w). Authentic samples of levan (L8647 from Erwinia herbicola; Sigma-Aldrich), sucrose, fructose, and glucose were dissolved in 4% (w/v) H2SO4 to reach 0.5%, 2%, 1%, and 1% (w/w), respectively. The suspensions were hydrolyzed at 121 °C for 1 h and centrifuged at 20 000 × g for 5 min at 4 °C. The supernatant was filtered (pore size 0.2 µm) and analyzed by HPLC using an Aminex HPX-87H column (300 × 87 mm; Bio-Rad), a RID-10A detector (Shimazu,), an effluent of filtered and degassed 0.01N H2SO4, a flow rate of 0.6 ml/min, and a column temperature of 65.0 °C.
For TLC analysis, collected and weighed cells were suspended in 3% (v/v) trichloroacetic acid (TCA) to reach 14% (w/w). Authentic sample of levan was dissolved in 3% (v/v) TCA to reach 0.5% (w/v). Authentic samples of levan, fructose, and glucose were also dissolved in water to reach 0.5% (w/w). The suspensions in 3% (v/v) TCA were hydrolyzed at 55 °C for 15 minCitation34,Citation35 and centrifuged at 20 000 × g for 5 min at 4 °C. The supernatant (5 µl) and other authentic sample (5 µl) were spotted on TLC silica gel 60 F254 (Merck KGaA) and developed in a solvent system consisting of butanol-acetate-water (3:3:2 v/v/v). Levan, fructose, and fructosyl derivatives were specifically detected using resorcinol and thiourea as described.Citation34
Isolation of levan from liquid culture
G. diazotrophicus Pal5 cells were precultured in liquid C2-NaCl medium, washed with 0.9% NaCl as above, and inoculated to OD600 of 0.1 in 30 ml of fresh liquid high P medium in a 300 ml Erlenmeyer flask. Cells were cultivated at 150 strokes per min at 30 °C for 48 h and supernatant was obtained by centrifugation at 20 000 g for 5 min. Levan was isolated from the supernatant as described previously.Citation19 Briefly, levan was precipitated with 2 volumes of ethanol 99.5%, collected with centrifugation at 20 000 g for 20 min, washed once with 66% ethanol, and freeze-dried. The dried material was weighed and a portion was dissolved in 4% (w/v) H2SO4 to reach 1% (w/w), hydrolyzed, and analyzed by HPLC as above.
Determination of phosphate concentration in sugarcane juice
Sugarcanes grown in Okinawa were purchased from Ryuka Shoji (Okinawa, Japan), Hatsuhino (Okinawa, Japan), and Ryukyu farm (Okinawa, Japan). Phosphate concentrations were determined in three sugarcanes from each company (a1, a2, a3, b1, b2, b3, c1, c2, and c3) using a previously described method.Citation36
Isolation of bacteria from sugarcane juice
Sugarcane juice (1 µl) was streaked on solid high P medium containing 5 ml of 0.5% bromothymol blue in 0.2 N KOH in 1 L of medium. Each colony was purified by streaking twice on solid low P medium. Genomic DNA was isolated from bacteria and the 16S rDNA gene was amplified using primers 13 and 14 (). Sequence analysis of the amplified fragment was conducted using primers 13, 14, 15, and 16. rDNA sequences were analyzed by BLAST searchCitation37 using the NCBI 16S rRNA sequence database. Bacteria were tentatively identified based on the highest max score.
Tolerance to hydrogen peroxide
G. diazotrophicus Pal5 was precultured in C2-NaCl, washed 3 times with 0.9% NaCl, and diluted to OD600 of 1.0. The cell suspension (2 µl) was spotted on solid media containing Na2HPO4 as a sole phosphate source. The pH was adjusted to 5.0 to match that of sugarcane juice.
Additional material
Download Zip (243.3 KB)Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Acknowledgments
This study was supported by Grants-in-Aid for challenging Exploratory Research (to K.M.; 24658076, 22658026), and in part by the Funding Program for Next Generation World-Leading Researchers (NEXT program) (to S.K.).
References
- Bhattacharjee RB, Singh A, Mukhopadhyay SN. Use of nitrogen-fixing bacteria as biofertiliser for non-legumes: prospects and challenges. Appl Microbiol Biotechnol 2008; 80:199 - 209; http://dx.doi.org/10.1007/s00253-008-1567-2; PMID: 18600321
- Berg G. Plant-microbe interactions promoting plant growth and health: perspectives for controlled use of microorganisms in agriculture. Appl Microbiol Biotechnol 2009; 84:11 - 8; http://dx.doi.org/10.1007/s00253-009-2092-7; PMID: 19568745
- Cocking E, Stone P, Davey M. Intracellular colonization of roots of Arabidopsis and crop plants by Gluconacetobacter diazotrophicus.. In Vitro Cell Dev Biol Plant 2006; 42:74 - 82; http://dx.doi.org/10.1079/IVP2005716
- Cavalcante V, Dobereiner J. A new acid-tolerant nitrogen-fixing bacterium associated with sugarcane. Plant Soil 1988; 108:23 - 31; http://dx.doi.org/10.1007/BF02370096
- Paula MA, Reis VM, Döbereiner J. Interactions of Glomus clarum with Acetobacter diazotrophicus in infection of sweet potato (Ipomoea batatas), sugarcane (Saccharum spp.), and sweet sorghum (Sorghum vulgare). Biol Fertil Soils 1991; 11:111 - 5; http://dx.doi.org/10.1007/BF00336374
- Jimenez-Salgado T, Fuentes-Ramirez LE, Tapia-Hernandez A, Mascarua-Esparza MA, Martinez-Romero E, Caballero-Mellado J. Coffea arabica L., a new host plant for Acetobacter diazotrophicus, and isolation of other nitrogen-fixing acetobacteria. Appl Environ Microbiol 1997; 63:3676 - 83; PMID: 9293018
- Reis VM, Olivares FL, Döbereiner J. Improved methodology for isolation of Acetobacter diazotrophicus and confirmation of its endophytic habitat. World J Microbiol Biotechnol 1994; 10:401 - 5; http://dx.doi.org/10.1007/BF00144460; PMID: 24421085
- Tapia-Hernández A, Bustillos-Cristales MR, Jiménez-Salgado T, Caballero-Mellado J, Fuentes-Ramírez LE. Natural endophytic occurrence of Acetobacter diazotrophicus in pineapple plants. Microb Ecol 2000; 39:49 - 55; http://dx.doi.org/10.1007/s002489900190; PMID: 10790517
- Bastián F, Cohen A, Piccoli P, Luna V, Bottini R, Baraldi R, et al. Production of indole-3-acetic acid and gibberellins A1 and A3 by Acetobacter diazotrophicus and Herbaspirillum seropedicae in chemically-defined culture media. Plant Growth Regul 1998; 24:7 - 11; http://dx.doi.org/10.1023/A:1005964031159
- Fuentes-Ramirez LE, Jimenez-Salgado T, Abarca-Ocampo IR, Caballero-Mellado J. Acetobacter diazotrophicus, an indoleacetic acid producing bacterium isolated from sugarcane cultivars of México. Plant Soil 1993; 154:145 - 50; http://dx.doi.org/10.1007/BF00012519
- Lee S, Flores-Encarnación M, Contreras-Zentella M, Garcia-Flores L, Escamilla JE, Kennedy C. Indole-3-acetic acid biosynthesis is deficient in Gluconacetobacter diazotrophicus strains with mutations in cytochrome c biogenesis genes. J Bacteriol 2004; 186:5384 - 91; http://dx.doi.org/10.1128/JB.186.16.5384-5391.2004; PMID: 15292139
- Piñón D, Casas M, Blanch M, Fontaniella B, Blanco Y, Vicente C, Solas MT, Legaz ME. Gluconacetobacter diazotrophicus, a sugar cane endosymbiont, produces a bacteriocin against Xanthomonas albilineans, a sugar cane pathogen. Res Microbiol 2002; 153:345 - 51; http://dx.doi.org/10.1016/S0923-2508(02)01336-0; PMID: 12234008
- Saravanan VS, Kalaiarasan P, Madhaiyan M, Thangaraju M. Solubilization of insoluble zinc compounds by Gluconacetobacter diazotrophicus and the detrimental action of zinc ion (Zn2+) and zinc chelates on root knot nematode Meloidogyne incognita.. Lett Appl Microbiol 2007; 44:235 - 41; http://dx.doi.org/10.1111/j.1472-765X.2006.02079.x; PMID: 17309498
- Intorne AC, de Oliveira MV, Lima ML, da Silva JF, Olivares FL, de Souza Filho GA. Identification and characterization of Gluconacetobacter diazotrophicus mutants defective in the solubilization of phosphorus and zinc. Arch Microbiol 2009; 191:477 - 83; http://dx.doi.org/10.1007/s00203-009-0472-0; PMID: 19340412
- Baldani J, Caruso L, Baldani V, Goi S, Dobereiner J. Recent advances in BNF with non-legume plants. Soil Biol Biochem 1997; 29:911 - 22; http://dx.doi.org/10.1016/S0038-0717(96)00218-0
- Pan B, Vessey JK. Response of the endophytic diazotroph Gluconacetobacter diazotrophicus on solid media to changes in atmospheric partial O(2) pressure. Appl Environ Microbiol 2001; 67:4694 - 700; http://dx.doi.org/10.1128/AEM.67.10.4694-4700.2001; PMID: 11571174
- Yoch DC, Pengra RM. Effect of amino acids on the nitrogenase system of Klebsiella pneumoniae.. J Bacteriol 1966; 92:618 - 22; PMID: 5922536
- Meneses CHSG, Rouws LFM, Simões-Araujo JL, Vidal MS, Baldani JI. Exopolysaccharide production is required for biofilm formation and plant colonization by the nitrogen-fixing endophyte Gluconacetobacter diazotrophicus.. Mol Plant Microbe Interact 2011; 24:1448 - 58; http://dx.doi.org/10.1094/MPMI-05-11-0127; PMID: 21809982
- Hernandez L, Arrieta J, Menendez C, Vazquez R, Coego A, Suarez V, Selman G, Petit-Glatron MF, Chambert R. Isolation and enzymic properties of levansucrase secreted by Acetobacter diazotrophicus SRT4, a bacterium associated with sugar cane. Biochem J 1995; 309:113 - 8; PMID: 7619044
- Arrieta J, Hernández L, Coego A, Suárez V, Balmori E, Menéndez C, Petit-Glatron MF, Chambert R, Selman-Housein G. Molecular characterization of the levansucrase gene from the endophytic sugarcane bacterium Acetobacter diazotrophicus SRT4. Microbiology 1996; 142:1077 - 85; http://dx.doi.org/10.1099/13500872-142-5-1077; PMID: 8704949
- Venieraki A, Dimou M, Vezyri E, Kefalogianni I, Argyris N, Liara G, Pergalis P, Chatzipavlidis I, Katinakis P. Characterization of nitrogen-fixing bacteria isolated from field-grown barley, oat, and wheat. J Microbiol 2011; 49:525 - 34; http://dx.doi.org/10.1007/s12275-011-0457-y; PMID: 21887633
- Velázquez-Hernández ML, Baizabal-Aguirre VM, Cruz-Vázquez F, Trejo-Contreras MJ, Fuentes-Ramírez LE, Bravo-Patiño A, Cajero-Juárez M, Chávez-Moctezuma MP, Valdez-Alarcón JJ. Gluconacetobacter diazotrophicus levansucrase is involved in tolerance to NaCl, sucrose and desiccation, and in biofilm formation. Arch Microbiol 2011; 193:137 - 49; http://dx.doi.org/10.1007/s00203-010-0651-z; PMID: 21103984
- Bezzate S, Aymerich S, Chambert R, Czarnes S, Berge O, Heulin T. Disruption of the Paenibacillus polymyxa levansucrase gene impairs its ability to aggregate soil in the wheat rhizosphere. Environ Microbiol 2000; 2:333 - 42; http://dx.doi.org/10.1046/j.1462-2920.2000.00114.x; PMID: 11200435
- Geier G, Geider K. Characterization and influence on virulence of the levansucrase gene from the fireblight pathogen Erwinia amylovora.. Physiol Mol Plant Pathol 1993; 42:387 - 404; http://dx.doi.org/10.1006/pmpp.1993.1029
- Schroeder VA, Michalek SM, Macrina FL. Biochemical characterization and evaluation of virulence of a fructosyltransferase-deficient mutant of Streptococcus mutans V403. Infect Immun 1989; 57:3560 - 9; PMID: 2807537
- Lamb C, Dixon RA. The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol 1997; 48:251 - 75; http://dx.doi.org/10.1146/annurev.arplant.48.1.251; PMID: 15012264
- Alquéres S, Meneses C, Rouws L, Rothballer M, Baldani I, Schmid M, Hartmann A. The bacterial superoxide dismutase and glutathione reductase are crucial for endophytic colonization of rice roots by Gluconacetobacter diazotrophicus PAL5. Mol Plant Microbe Interact 2013; 26:937 - 45; http://dx.doi.org/10.1094/MPMI-12-12-0286-R; PMID: 23634840
- Serrato RV, Meneses CH, Vidal MS, Santana-Filho AP, Iacomini M, Sassaki GL, Baldani JI. Structural studies of an exopolysaccharide produced by Gluconacetobacter diazotrophicus Pal5. Carbohydr Polym 2013; 98:1153 - 9; http://dx.doi.org/10.1016/j.carbpol.2013.07.025; PMID: 23987457
- Schallmey M, Singh A, Ward OP. Developments in the use of Bacillus species for industrial production. Can J Microbiol 2004; 50:1 - 17; http://dx.doi.org/10.1139/w03-076; PMID: 15052317
- Yamamoto Y, Takahashi Y, Kawano M, Iizuka M, Matsumoto T, Saeki S, Yamaguchi H. In vitro digestibility and fermentability of levan and its hypocholesterolemic effects in rats. J Nutr Biochem 1999; 10:13 - 8; http://dx.doi.org/10.1016/S0955-2863(98)00077-1; PMID: 15539245
- Kim KH, Chung CB, Kim YH, Kim KS, Han CS, Kim CH. Cosmeceutical properties of levan produced by Zymomonas mobilis.. J Cosmet Sci 2005; 56:395 - 406; PMID: 16538295
- Rodrigues Neto J, Malavolta V, Victor O. Meio simples para o isolamento e cultivo de Xanthomonas campestris pv. Citri Tipo B. Summa Phytopathol 1986; 12:16
- Teixeira KRS, Wülling M, Morgan T, Galler R, Zellermann E-M, Baldani JI, et al. Molecular analysis of the chromosomal region encoding the nifA and nifB genes of Acetobacter diazotrophicus.. FEMS Microbiol Lett 1999; 176:301 - 9; http://dx.doi.org/10.1111/j.1574-6968.1999.tb13676.x
- Muro AC, Rodriguez E, Abate CM, Sineriz F. Identification in TLC of fructose and fructosyl derivatives in levan and sugar mixtures with resorcinol and thiourea. Folia Microbiol (Praha) 1999; 44:647 - 9; http://dx.doi.org/10.1007/BF02825655
- Dogsa I, Brloznik M, Stopar D, Mandic-Mulec I. Exopolymer diversity and the role of levan in Bacillus subtilis biofilms. PLoS One 2013; 8:e62044; http://dx.doi.org/10.1371/journal.pone.0062044; PMID: 23637960
- Chen P Jr.. Toribara Tt, Warner H. Microdetermination of phosphorus. Anal Chem 1956; 28:1756 - 8; http://dx.doi.org/10.1021/ac60119a033
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol 1990; 215:403 - 10; PMID: 2231712