Abstract
Virotherapy on the basis of oncolytic vaccinia virus (VACV) strains is a novel approach for cancer therapy. In this study we describe for the first time the use of dynamic boolean modeling for tumor growth prediction of vaccinia virus GLV-1h68-injected canine tumors including canine mammary adenoma (ZMTH3), canine mammary carcinoma (MTH52c), canine prostate carcinoma (CT1258), and canine soft tissue sarcoma (STSA-1). Additionally, the STSA-1 xenografted mice were injected with either LIVP 1.1.1 or LIVP 5.1.1 vaccinia virus strains.
Antigen profiling data of the four different vaccinia virus-injected canine tumors were obtained, analyzed and used to calculate differences in the tumor growth signaling network by type and tumor type. Our model combines networks for apoptosis, MAPK, p53, WNT, Hedgehog, TK cell, Interferon, and Interleukin signaling networks. The in silico findings conform with in vivo findings of tumor growth.
Boolean modeling describes tumor growth and remission semi-quantitatively with a good fit to the data obtained for all cancer type variants. At the same time it monitors all signaling activities as a basis for treatment planning according to antigen levels. Mitigation and elimination of VACV- susceptible tumor types as well as effects on the non-susceptible type CT1258 are predicted correctly. Thus the combination of Antigen profiling and semi-quantitative modeling optimizes the therapy already before its start.
Introduction
Cancer is among the top killer diseases in domestic and feral dogs.Citation1-Citation5 Incidence of canine cancer ranges from 1 to 2% in normal population and cancer currently accounts for about half of the deaths of animals older than 10 years.Citation1,Citation6
Standard treatment of canine cancer patients consists of invasive surgery in combination with radiation and chemotherapy. However, the available treatment options for canine patients with advanced-stage disease are limited and the prognosis for such patients is very poor. Virotherapy using oncolytic viruses (OVs) is one promising new strategy for cancer therapy. OVs preferentially infect and lyse cancer cells, without causing excessive damage to surrounding healthy tissue, and initiate tumor-specific immunity. Oncolytic viruses including various human and canine adenoviruses, canine distemper virus, and vaccinia virus strains have been successfully tested for canine cancer therapy in pre-clinical settings.Citation7,Citation8
Several vaccinia virus strains such as GLV-1h68, LIVP 1.1.1, LIVP 5.1.1, LIVP 6.1.1, and GLV-1h109 were successful used for treatment of canine cancer in pre-clinical studies.Citation9-Citation12
In this work we are looking for a way to quickly determine the effectiveness of viral vaccinia treatment against different types of canine tumors. To this end we will be using antigen quantity data from tumor samples of xenografted nude mice in order to determine a distinct signaling pattern for each of the tumor types and viral strains for later application in canine tumor therapy.
Results
In order to estimate the effectiveness of VACV- treatment in silico of the different tumor types ZMTH3, STSA-1, MTH52c, and CT1258 we decided to split these four types into two groups: in vivo responders to VACV- treatment (ZMTH3, STSA-1, and MTH52c) and in vivo non-responders (CT1258).
In order to obtain accurate immune-related protein antigen profiles, 21 or 42 days after virus or PBS treatment, three mice from each group were sacrificed and mouse immune-related protein antigen profiles were obtained. The antigen profiling data was used to calculate correlates of antigen quantities before and after treatment with VACV. These correlates where then directly applied as coefficients of input signal strengths for the input nodes of the model (see , yellow boxes).
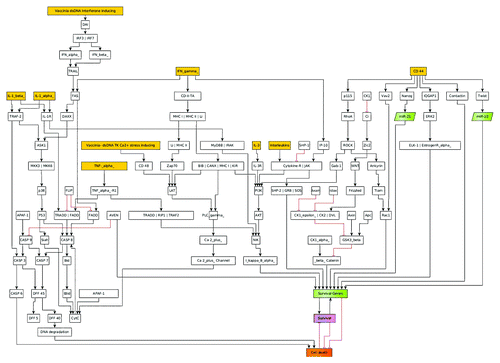
Figure 1. This figure depicts the general layout of the signaling model used to calculate the oncolytic virus effects on different types of canine cancers. In this model the apoptosis, MAPK, p53, WNT, Hedgehog, TK cell mediated cell death, IFN, and Interleukin signaling, as well as mitochondrial Ca2+- signaling are included. Yellow boxes denote the possible input signals for the models; green boxes denote genes with anti-apoptotic properties. The outcome of the model is given in the signaling strength of the purple (survival of cancer cell) and orange (apoptosis of cancer cell) boxes.
By this approach, we were able to determine differences in tumor growth of the different xenografts. In silico effects are similar to in vivo effects—although the calculated signal strength for tumor apoptosis in silico is not equal to the observed remission of tumor size in vivo: the calculation only gives information if the tumor goes into remission, but there is no information as to how fast this happens in vivo.
In order to train the models to the in vivo findings we set the proliferation rate—in the case of non-treated tumors—to always be higher than the tumor remission (see ). These fitted models were set to have a proliferation signal strength of 53% and 47% apoptosis signaling strength accordingly. These values were chosen to be well above and/or below the maximum standard error of the calculations of 1.5%. The model was then used to calculate the differences in the response of the different tumor types to VACV.
Figure 2. Shown here are models and/or ratios of tumor survival and apoptosis of the canine, MTH52c, STSA-1, and ZMTH3 tumors in nude mice. In order to estimate the differences between tumor survival and apoptosis, the polynomial area of the diagrams were calculated. Without viral therapy the tumors are in indefinite proliferation.
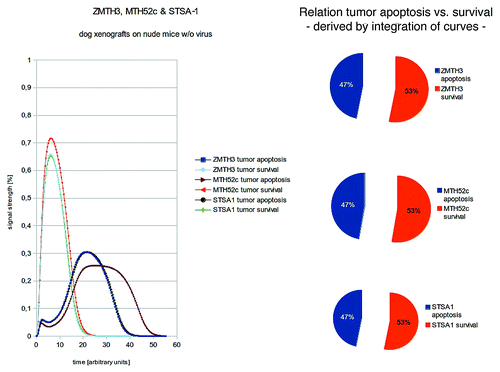
In order to estimate the differences between tumor survival and apoptosis, the polynomial area of the diagrams were calculated.
ZMTH3, STSA-1, and MTH52c
Calculations of signaling networks depending on correlations of tumor growth and remission in canine tumors MTH52c, STSA-1, and ZMTH3 with and without viral treatment show that:
ZMTH3 and STSA-1 are both good responders to VACV treatment in vivo.
STSA-1 responds best to LIVP 1.1.1 in silico as well as in vivo.
MTH52c is less responsive to viral treatment in vivo as well as in silico.
Without viral treatment, the xenografted tumors were modeled to be in indefinite proliferation (). As shown here, there are distinct differences in the actual signal strength for cell death and cell survival. After mapping the distinct signals after viral treatment and emulation of the innate immune systems response to tumorous cells, all three tumor types are in remission in silico () as well as in vivo.Citation13-Citation15
Figure 3. Models and/or ratios of tumor survival and apoptosis of the canine MTH52c, STSA-1, and ZMTH3 tumors in nude mice after therapy with different vaccinia virus strains. Again the polynomial areas of the diagrams were calculated. ZMTH3 and MTH52c both react very well to VACV- treatments and show tumor apoptosis that conform with in vivo findings. STSA-1 was treated with different virus strains (GLV-1h68, LIVP 1.1.1, and LIVP 5.1.1) and shows the highest tumor apoptosis when treated with LIVP 1.1.1- again in full agreement with the in vivo findings.
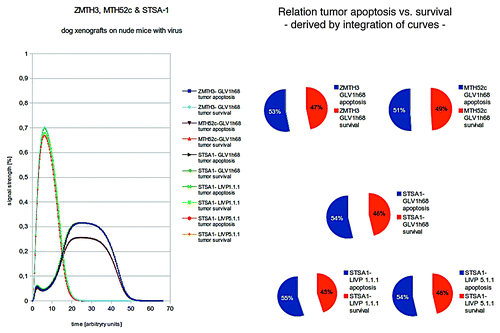
ZMTH3 and MTH52c both react very well to VACV-treatments and show tumor apoptosis that conform with in vivo findings. STSA-1 was treated with different virus strains (GLV-1h68, LIVP 1.1.1, and LIVP 5.1.1) and shows the highest tumor apoptosis when treated with LIVP 1.1.1- again in full agreement with the in vivo findings. Therefore this data driven model adaptation delivers a correct quantitative description of tumor growth and remission.
CT1258
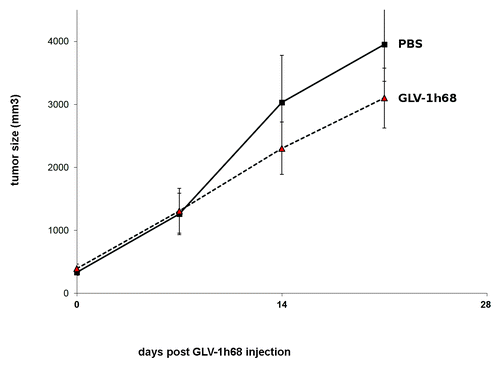
It was shown by Fork et al.Citation16 that the canine prostate carcinoma CT1258 cells are highly proliferating in vivo (). As shown in , the GLV-1h68-virus treatment did not lead to significant differences in tumor growth between PBS controls and all virus-treated mice (P > 0.05). Therefore, this tumor type seems to be non-responder to virus treatment under these experimental conditions. In addition, under cell culture conditions, an abundance of CD44 has additionally been described previously for CT1258.Citation17 To incorporate this into our models, we also modeled the effects of this over-expression of CD44 onto the cell survival and apoptosis signaling networks. For CT1258 we simulated the following conditions:
Figure 4. Growth of canine prostate carcinoma tumors in virus- and mock-treated mice. Groups of CT1258 tumor-bearing nude mice (n = 5) were either treated with a single dose of 5 × 106 pfu GLV-1h68 or with PBS (mock control). Tumor size was measured twice a week. There were no significant differences between groups (P > 0.05).
Tumor growth without virus treatment
Tumor growth with VACV treatment.
Again the polynomial areas of the diagrams were calculated. Our in silico modeling shows a highly proliferating tumor in the case of no treatment. Only in the case of vaccinia virus therapy, the tumor growth is reduced, but the tumor is still very well in proliferation ().
Figure 5. Two different treatments of CT1258 are compared: no therapy vs. VACV treatment. Again the polynomial areas of the diagrams were calculated. CT1258 is shown to be highly aggressive in vivo. Our in silico modeling shows a highly proliferating tumor in the case of no treatment.
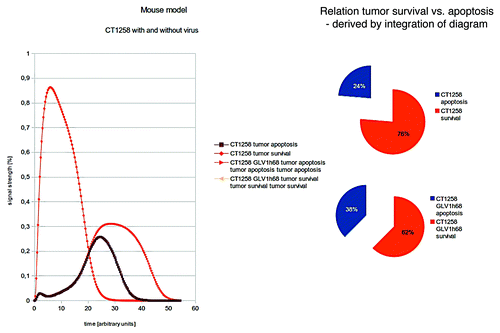
The findings of the in silico modeling are consistent with the in vivo findings ( and ) of a highly proliferating tumor even when VACV-treatment is supplied.
Discussion
In this work we establish a novel way to predict the effects of VACV-treatment on tumor growth in four different canine xenograft models. We were able to fit antigen profiling data directly onto the models and predict the outcome of VACV-therapy. Different types of data improve always different aspects of modeling, the accurate immune-related protein antigen profiles were critically to improve the signaling network, however, similarly high resolution metabolite profiling would resolve metabolic issues, for instance how far VACV virus replication profits from glycolytic state (typical for proliferating cancer states) and how redox stress and metabolic pathways determine the oncolytic effect of VACV. Metabolic profiling was not used in this approach as the focus was specifically on the signaling cascades of immune-related networks. To this end the antigen profiling data gives more accurate results for a narrower selection of compounds of interest. Detailed quantitative models are also possible regarding the metabolism if for this detailed concentration changes for a number of metabolites are available. Metabolic profiling will be essential for such an approach to obtain sufficient metabolite kinetics and future work will obtain such data. The result of such metabolic models will give the differences in the enzymatic fluxes of the different cancer strains.
For the treatment of canine cancer patients, these models may be used to correctly predict the tumor dependent outcome of the VACV-therapy according to antigen data. Moreover, a generation of personalized xenografts might be possible.
In future work we want to expand these predictive qualities of the models even further by going back to points in time much closer to the injection with VACV then the time-frame used for the mice xenografts of this work. This will enable us to be able to even more quickly determine the success of treatment.
We also want to explore the possibility of designing a CD44- suppressing VACV strain in order to attack CT1258 more effectively, as this difference in the CD44 expression is calculated to be one of the reasons as to why this tumor type is highly aggressive and resistant to VACV- treatment.
These methods are very well suited to capture signaling processes in cellular signaling networks if only limited information is available. Though cellular signaling has been described a lot by such models this is the first publication applying the technique to elucidate action, inhibition and modification of cellular signaling under such a designed virus approach. The results not only look interesting but also allow to improve therapeutic potential of the oncolytic virus including tumor or individual canine-specific modifications. There are, however, certain limitations: We focused on signaling and had to deal with limited data, in particular, detailed kinetics were not available. We hence applied a semi-quantitative approach where e-functions interpolate between full ON or OFF states of signaling nodes. If instead detailed time-courses for key signaling and read-out components are available, a fully quantitative description is possible, capturing also kinetics of key players by fine adjusted parameter values for their concentrations and interactions instead using exponential interpolation by generic algorithms according to Boolean network state and input from neighbor nodes.
More specific information as to the signal strengths in the apoptosis signaling networks is another area where we want to improve our modeling of the regulatory effects. Numerous studies are available describing apoptosis pathways and modifications. However, modification of apoptosis pathways by different virus strains is complex and will be considered in detail for our oncolytic virus approach in an iterative process of modeling and experiment to further improve our approach also in this respect.
Materials and Methods
Vaccinia virus strains
LIVP 1.1.1 and LIVP 5.1.1 were isolated from a wild type stock of Lister strain of vaccinia virus (Lister strain, Institute of Viral Preparations [LIVP], Moscow, Russia) and represent “native” viruses for which no genetic manipulations were conducted.Citation13,Citation18,Citation19 GLV-1h68 was also developed from the Lister strain by inserting three expression cassettes encoding Renilla luciferase-Aequorea green fluorescent protein fusion (Ruc-GFP), LacZ, and β-glucuronidase into the F14.5L, J2R (thymidine kinase) and A56R (hemagglutinin) loci of the viral genome, respectively.Citation20
Vaccinia virus-mediated therapy of canine xenografts
The vaccinia virus-mediated effects on the tumor growth of ZMTH3, MTH52c, and (STSA-1) xenografts were already described.Citation8,Citation14,Citation15
The CT1258 canine tumors were generated by implanting canine prostate carcinoma CT1258 cells subcutaneously into the right hind leg of 6 to 8 weeks old female nude mice (Hsd: Athymic Nude-Foxn1nu; Harlan, Netherlands.Citation21 Tumor growth was monitored twice weekly in two dimensions using a digital caliper. Tumor volume was calculated as [(length × width2)/2]. On day 8, when tumor volume reached approximately 400 mm3, groups of mice (n = 5) were injected intravenously (i.v.) either with 5 × 106 pfu of GLV-1h68 virus or PBS (control) into the tail vein.
Antigen profiling data
At 21 or 42 d after virus or PBS treatment, three mice from each group were sacrificed. Tumors were excised, weighted, and homogenized using FastPrep FP120 Cell Disruptor (BIO 101, Qbiogene) at a speed of 6000 g for 20 s (three times). Samples were then centrifuged at 10 000 g at 4 °C for 5 min and the supernatants were analyzed for mouse immune-related protein antigen profiling by Multi-Analyte Profiles (mouse MAPs; Rules Based Medicine) using antibody linked beads. Results were normalized based on total protein concentration.
Dynamic modeling
Identification of the most relevant antigens from the general apoptosis and tumor survival signaling networks was done, as well as key molecular components for all considered cascades were incorporated into a machine readable CellDesigner 3.5.2 (Funahashi et al.Citation22) network model, which takes their respective logical connectivity into account. In order to present the model in a more appealing way, we refined the layout in the yED- Graph Editor (yWorks, Tübingen, Germany, yED Version 3.11.1Citation23). It includes the apoptosis, MAPK, p53, WNT, Hedgehog, TK cell mediated cell death, IFN, and Interleukin signaling, as well as mitochondrial Ca2+- signaling. (; Table S1 gives details on components). Custom scripting for boolean semi-quantitative modeling in SQUAD (Di Cara et al.Citation24) was then set up using the topology information and logical connectivity from the CellDesigner model. Semi-quantitative modeling is reached by the SQUAD- algorithm applying e-functions to interpolate between “ON” and “OFF” boolean states. The simulations model the effects of different inputs into the model (, yellow boxes) and the systems response (, purple and orange).
Antigen profiling data was obtained for CT1258, MTH52c, STSA-1, and ZMTH3 tumors with and without viral treatment. Input data for SQUAD- modeling consists of Interleukin, TNF-α, and IFN-γ antigens.
To simulate signaling networks from antigen quantity, the differences in measured antigen quantities were compared before and after viral treatment. The resulting coefficient was then used to define the starting signal strength in the models. The models were also calibrated to simulate tumor proliferation in non-treated tumor cells. For tumor cells that underwent viral treatment, the same calibration as in the non-treated cells was used, but the signaling of natural killer cells was, additionally to the different signaling inputs derived from antigen quantity, activated to emulate the effects of the innate immune system of naked mice on the tumor cells.
Differences in signal strength leading to tumor growth or remission were directly compared as well as by their integrals.
| Abbreviations: | ||
| VACV | = | Vaccinia virus |
| ZMTH3 | = | canine mammary adenoma cell line |
| MTH52c | = | canine mammary carcinoma cell line |
| CT1258 | = | canine prostate carcinoma cell line |
| STSA-1 | = | canine soft tissue sarcoma cell line |
| LIVP 1.1.1 | = | Lister strain of vaccinia virus |
| LIVP 5.1.1 | = | Lister strain of vaccinia virus |
| TNF | = | tumor necrosis factor |
| IFN | = | Interferon |
Additional material
Download Zip (682.4 KB)Disclosure of Potential Conflicts of Interest
I.G. and A.A.S. are employees and shareholders of Genelux Corporation, San Diego, USA. A.C. and M.A. were supported by grants of Genelux Corporation awarded to University of Würzburg. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions
A.C. provided boolean modeling for the tumor types as well as data analysis to fit the antigen profiling data to the models. I.G. and M.A. did the in vivo testing of VACV treatments for the analyzed tumor types and generated the antigen profiling data. I. N. provided the canine cancer cells lines. T.D. provided expert knowledge and the bioinformatics facilities. A.A.S. provided expert knowledge of VACV treatments and funding.
References
- Merlo DF, Rossi L, Pellegrino C, Ceppi M, Cardellino U, Capurro C, Ratto A, Sambucco PL, Sestito V, Tanara G, et al. Cancer incidence in pet dogs: findings of the Animal Tumor Registry of Genoa, Italy. J Vet Intern Med 2008; 22:976 - 84; http://dx.doi.org/10.1111/j.1939-1676.2008.0133.x; PMID: 18564221
- Dorn ER. Epidemiology of canine and feline tumors. J Am Anim Hosp Assoc 1976; 12:307 - 12
- Gobar GM, Case JT, Kass PH. Program for surveillance of causes of death of dogs, using the Internet to survey small animal veterinarians. J Am Vet Med Assoc 1998; 213:251 - 6; PMID: 9676598
- Bronson RT. Variation in age at death of dogs of different sexes and breeds. Am J Vet Res 1982; 43:2057 - 9; PMID: 6891194
- Foundation MA. In Companion Animal News, Animal Health Survey. Morris Animal Foundation 1998, 2005.
- Kelsey JL, Moore AS, Glickman LT. Epidemiologic studies of risk factors for cancer in pet dogs. Epidemiol Rev 1998; 20:204 - 17; http://dx.doi.org/10.1093/oxfordjournals.epirev.a017981; PMID: 9919439
- Patil SS, Gentschev I, Nolte I, Ogilvie G, Szalay AA. Oncolytic virotherapy in veterinary medicine: current status and future prospects for canine patients. J Transl Med 2012; 10:3; http://dx.doi.org/10.1186/1479-5876-10-3; PMID: 22216938
- Gentschev I, Patil SS, Petrov I, Cappello J, Adelfinger M, Szalay AA. Oncolytic virotherapy of canine and feline cancer. Viruses 2014; 6:2122 - 37; http://dx.doi.org/10.3390/v6052122; PMID: 24841386
- Whilding LM, Archibald KM, Kulbe H, Balkwill FR, Öberg D, McNeish IA. Vaccinia virus induces programmed necrosis in ovarian cancer cells. Mol Ther 2013; 21:2074 - 86; http://dx.doi.org/10.1038/mt.2013.195; PMID: 23985697
- Patil SS, Gentschev I, Adelfinger M, Donat U, Hess M, Weibel S, Nolte I, Frentzen A, Szalay AA. Virotherapy of canine tumors with oncolytic vaccinia virus GLV-1h109 expressing an anti-VEGF single-chain antibody. PLoS One 2012; 7:e47472; http://dx.doi.org/10.1371/journal.pone.0047472; PMID: 23091626
- Chen NG, Yu YA, Zhang Q, Szalay AA. Replication efficiency of oncolytic vaccinia virus in cell cultures prognosticates the virulence and antitumor efficacy in mice. J Transl Med 2011; 9:164; http://dx.doi.org/10.1186/1479-5876-9-164; PMID: 21951588
- Gentschev I, Donat U, Hofmann E, Weibel S, Adelfinger M, Raab V, Heisig M, Chen N, Yu YA, Stritzker J, et al. Regression of human prostate tumors and metastases in nude mice following treatment with the recombinant oncolytic vaccinia virus GLV-1h68. J Biomed Biotechnol 2010; 2010:489759; http://dx.doi.org/10.1155/2010/489759; PMID: 20379368
- Gentschev I, Adelfinger M, Josupeit R, Rudolph S, Ehrig K, Donat U, Weibel S, Chen NG, Yu YA, Zhang Q, et al. Preclinical evaluation of oncolytic vaccinia virus for therapy of canine soft tissue sarcoma. PLoS One 2012; 7:e37239; http://dx.doi.org/10.1371/journal.pone.0037239; PMID: 22615950
- Gentschev I, Stritzker J, Hofmann E, Weibel S, Yu YA, Chen N, Zhang Q, Bullerdiek J, Nolte I, Szalay AA. Use of an oncolytic vaccinia virus for the treatment of canine breast cancer in nude mice: preclinical development of a therapeutic agent. Cancer Gene Ther 2009; 16:320 - 8; http://dx.doi.org/10.1038/cgt.2008.87; PMID: 18949014
- Gentschev I, Ehrig K, Donat U, Hess M, Rudolph S, Chen N, Yu YA, Zhang Q, Bullerdiek J, Nolte I, et al. Significant Growth Inhibition of Canine Mammary Carcinoma Xenografts following Treatment with Oncolytic Vaccinia Virus GLV-1h68. J Oncol 2010; 2010:736907; http://dx.doi.org/10.1155/2010/736907; PMID: 20631910
- Fork MA, Murua Escobar H, Soller JT, Sterenczak KA, Willenbrock S, Winkler S, Dorsch M, Reimann-Berg N, Hedrich HJ, Bullerdiek J, et al. Establishing an in vivo model of canine prostate carcinoma using the new cell line CT1258. BMC Cancer 2008; 8:240; http://dx.doi.org/10.1186/1471-2407-8-240; PMID: 18706092
- Moulay M, Liu W, Willenbrock S, Sterenczak KA, Carlson R, Ngezahayo A, Murua Escobar H, Nolte I. Evaluation of stem cell marker gene expression in canine prostate carcinoma- and prostate cyst-derived cell lines. Anticancer Res 2013; 33:5421 - 31; PMID: 24324078
- Gentschev I, Patil SS, Adelfinger M, Weibel S, Geissinger U, Frentzen A, Chen NG, Yu YA, Zhang Q, Ogilvie G, et al. Characterization and evaluation of a new oncolytic vaccinia virus strain LIVP6.1.1 for canine cancer therapy. Bioengineered 2013; 4:84 - 9; http://dx.doi.org/10.4161/bioe.22462; PMID: 23093804
- Advani SJ, Buckel L, Chen NG, Scanderbeg DJ, Geissinger U, Zhang Q, Yu YA, Aguilar RJ, Mundt AJ, Szalay AA. Preferential replication of systemically delivered oncolytic vaccinia virus in focally irradiated glioma xenografts. Clin Cancer Res 2012; 18:2579 - 90; http://dx.doi.org/10.1158/1078-0432.CCR-11-2394; PMID: 22379115
- Zhang Q, Yu YA, Wang E, Chen N, Danner RL, Munson PJ, Marincola FM, Szalay AA. Eradication of solid human breast tumors in nude mice with an intravenously injected light-emitting oncolytic vaccinia virus. Cancer Res 2007; 67:10038 - 46; http://dx.doi.org/10.1158/0008-5472.CAN-07-0146; PMID: 17942938
- Harlan Laboratories [Internet]. Maasheseweg 87c, Venray 5804 AB, Netherlands: Hsd: Athymic Nude-Foxn1nu mice; c2014 [cited 2014 May 15]. Available from: http://www.harlan.com
- Funahashi A, Matsuoka Y, Jouraku A, Morohashi M, Kikuchi N, Kitano H. CellDesigner 3.5: A Versatile Modeling Tool for Biochemical Networks. Proc IEEE 2008; 96:1254 - 65; http://dx.doi.org/10.1109/JPROC.2008.925458
- yWorks GmbH [Internet]. Vor dem Kreuzberg 28, 72070 Tübingen, Germany: yED Graph Editor, Version 3.11.; c2014 [cited 2014 May 15]. Available from: http://www.yworks.com/en/products_yed_about.html
- Di Cara A, Garg A, De Micheli G, Xenarios I, Mendoza L. Dynamic simulation of regulatory networks using SQUAD. BMC Bioinformatics 2007; 8:462; http://dx.doi.org/10.1186/1471-2105-8-462; PMID: 18039375
