Abstract
Chondroitin sulfate is a major component of the extracellular matrix in both the central and peripheral nervous systems. Chondroitin sulfate is upregulated at injury, thus methods to promote neurite extension through chondroitin sulfate-rich matrices and synthetic scaffolds are needed. We describe the use of both chondroitin sulfate and a novel chondroitin sulfate-binding peptide to control the release of nerve growth factor. Interestingly, the novel chondroitin sulfate-binding peptide enhances the controlled release properties of the chondroitin sulfate gels. While introduction of chondroitin sulfate into a scaffold inhibits primary cortical outgrowth, the combination of chondroitin sulfate, chondroitin sulfate-binding peptide and nerve growth factor promotes primary cortical neurite outgrowth in chondroitin sulfate gels.
Introduction
Chondroitin sulfate (CS) is a glycosaminoglycan (GAG) found attached to a protein core to form a proteoglycan. Chondroitin sulfate proteoglycans (CSPGs) play an important role in the extracellular matrix in the central nervous system.Citation1 In neuronal development and regeneration, CSPGs modulate a wide range of activities from cell adhesion and division to synaptic plasticity and regeneration,Citation2,Citation3 and several studies have shown that the activity of CSPGs can be attributed to the sulfation pattern of the CS chains.Citation4-Citation6 Previous work has investigated the effects of the CS GAG in vitro both in solution or adsorbed onto a flat surface,Citation7-Citation10 but few have examined its effects on neurite outgrowth in three-dimensional gels.Citation6,Citation11,Citation12
CS-based biomaterials have been developed for a variety of applications, including cartilage tissue engineeringCitation13,Citation14 and wound healing.Citation15 In many of these studies, CS is modified either for covalent cross-linking for gel synthesis or for incorporation into the scaffold; however, the chemical modification of CS can interfere with potential binding sites and reduce CS bioactivity. Other systems physically entrap CS within the matrix, and diffusion of CS is controlled by the physical properties of the biomaterial.Citation16 Other GAGs have been incorporated into hydrogels as well, and in some cases, these have been added by integrating GAG-binding domains into the biomaterial. Sakiyama-Elbert et al. have developed an affinity-based system where heparin was incorporated in fibrin matrices through heparin-binding peptides.Citation17,Citation18 This group has also shown that growth factor activity can be regulated by GAG-binding through sequestration and localization of growth factor activity.Citation19 Sakiyama-Elbert et al. have improved neurite outgrowth both in vitroCitation20-Citation22 and in vivoCitation23 through affinity-based delivery of neurotrophins.
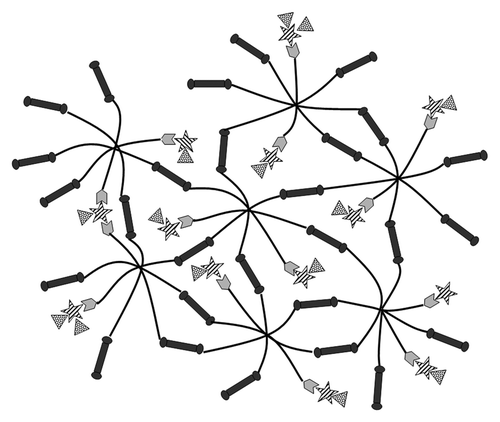
Our lab has developed a poly(ethylene glycol)(PEG)-co-peptide polysaccharide system that has tunable viscoelastic and biological properties, as seen in .Citation24-Citation27 In earlier studies, we incorporated heparin in the system in order to bind cell-penetrating peptides. In the current study, we have modified the material specifically to incorporate CS and take advantage of native interactions between CS and nerve growth factor (NGF) for controlled release. The mechanical properties of this material are controlled both through physical interactions of GAG-binding peptides, covalently bound to eight-arm PEG, with GAGs and through the cross-linking of eight-arm PEG (black lines) with bi-functional enzymatically degradable cross-linking peptides (dark gray dumbbells) that include an integrin-binding sequence (RGD). Unmodified CS (striped stars) is entrapped within the biomaterial through interactions with CS-binding peptides (light gray hexagons) conjugated to eight-arm PEG. Finally, CS provides binding sites for the incorporation of NGF (spotted triangles).
Figure 1. Affinity-based NGF delivery from PEG-co-peptide CS system. Eight-arm PEG (black lines) are modified with bi-functional cross-linking peptides (dark gray dumbbells) on 6 arms and CS-binding peptides (light gray hexagons) on 2 arms. CS (striped stars) interacts with CS-binding peptides and NGF (spotted triangles).
In earlier work, we demonstrated the viability of chondroitin-6-sulfate (C6S)-based scaffolds to support outgrowth of dorsal root ganglia (DRGs) in vitro.Citation28 Thus, this system has potential for use as a therapeutic implantable hydrogel to promote regeneration of neurons in traumatic root avulsion brachial plexus injuries. However, regeneration in these injuries will require both peripheral and central nerve growth, and previous studies have revealed that C6S inhibits the regeneration of central neurons.Citation29 This lack of central nervous system neuronal growth will likely prevent successful reintegration of the central and peripheral nervous systems if a C6S-based material were implanted in an in vivo model. Incorporation of the C6S-binding peptide described in previous work and investigated in the current work may help block these inhibitory signals and promote recovery after traumatic root avulsion brachial plexus injuries.Citation29,Citation30
To validate, in vitro, the potential use of this system as a therapy, we investigated the controlled release of NGF from this C6S-based biomaterial. In addition, we investigated the effects of NGF release on primary cortical neurite outgrowth. Controlled release of NGF is achieved via non-covalent interactions between NGF, CS and CS-binding peptide. Neurite outgrowth was inhibited on gels that only included C6S, but this inhibition was overcome when NGF was incorporated into the gel.
Results
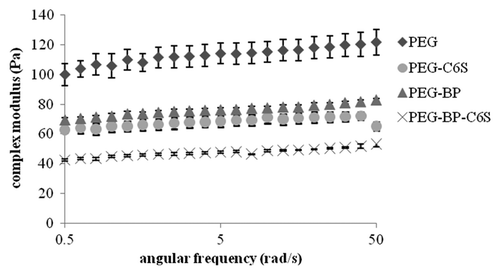
To investigate the effect peptide and CS incorporation into PEG gels had on the viscoelastic properties of gels, the compositions shown in were investigated using rheology. As negative controls, gels without C6S and/or BP (binding peptide) were tested. shows the complex modulus (G*) for the different gel compositions at 0.5–50 rad/s frequency and 0.5% strain. The PEG gel without C6S and BP (PEG) was the strongest, while the PEG gel with C6S and BP (PEG-BP-C6S) was the weakest. The two-way repeated measures ANOVA showed that the addition of C6S and BP significantly affected the viscoelastic properties of the gels.
Table 1. Gel compositions
Figure 2. Frequency sweep (0.5–500 rad/s) of gels at 0.5% strain. PEG gels without C6S or BP had the highest viscoelastic properties while gels with C6S and/or BP had significantly lower complex moduli. Mean ± SE.
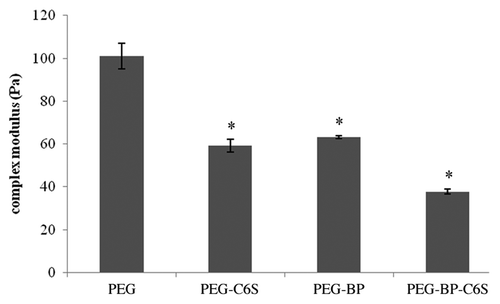
From the frequency sweep, 10 rad/s was chosen from the linear viscoelastic range, and a time sweep was performed at 0.5% strain for 6 min. shows the averaged complex modulus for the different gel compositions. At 10 rad/s and 0.5% strain, the PEG gel (100 Pa) was significantly (p < 0.05) stronger than all other gels with C6S and/or BP. The weakest gel (~38 Pa) contained both BP and C6S (PEG-BP-C6S) and was not statistically different (p > 0.05) from gels that contained either C6S (PEG-C6S) or BP (PEG-BP). These results demonstrate that PEG gels that contain either BP or C6S are significantly weaker than gels without BP or C6S.
Figure 3. Time sweep of PEG gels at 10 rad/s and 0.5% strain. PEG gels that contained C6S and/or BP had significantly lower complex moduli than gels without C6S or BP. Mean ± SE, *p < 0.05 different relative to PEG.
To demonstrate that inclusion of C6S would provide a controlled release mechanism, studies were done to investigate NGF release from the various gel compositions shown in . The amount of NGF released over 48 h was quantified with an ELISA kit. As negative controls, NGF release from gels without C6S and/or BP was monitored to determine if C6S and BP affected NGF release from PEG gels. The release profiles of the gels are shown in . PEG gels that contained only C6S (PEG-C6S) had the fastest NGF release, while gels that contained both BP and C6S had the slowest release profile (PEG-BP-C6S). The two-way ANOVA analysis showed that the addition of BP to PEG gels was a significant factor affecting NGF release, whereas C6S was not. shows the results from the post-hoc test to determine significance of NGF release between gels at different time points (0–48 h). At earlier time points (0–7 h), the amount of NGF released from the PEG-C6S gel is significantly greater than NGF released from gels with the BP (, light shading). At later time points (4–48 h), NGF release is significantly slower in PEG-BP-C6S than in all other gels (, dark shading). These results show that PEG gels that contain only BP have a slower NGF release profile when compared with PEG controls, while gels that contain both BP and C6S demonstrate the slowest NGF release profile.
Figure 4. NGF release profile of gels with and without BP and C6S. NGF release was monitored over 48 h. After 2 d, the gels were digested and the amount of NGF quantified. PEG gels that include BP and C6S had the slowest release while PEG gels that only had C6S had the fastest release. Mean ± SE.
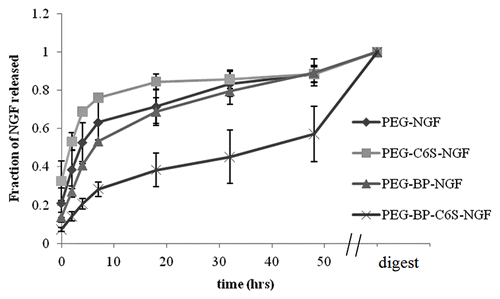
Table 2. Summary of post-hoc test of NGF release from different gels
To examine the effects of C6S, BP and controlled NGF release from the gels on neurite outgrowth, cortical neurons were cultured on PEG gels of different compositions () for 48 h. The average and maximum length and number of neurites was quantified for each neuron. Approximately 80–200 neurons were analyzed for each gel composition. shows (1) the average neurite length, (2) maximum neurite length (3) and number of neuritis for each gel composition. Controls consisted of neurons cultured on PEG gels that did not include C6S and/or NGF. PEG gels that included only C6S (PEG-C6S) had lower average/maximum neurite length and number of neurites compared with PEG gels without C6S (PEG-BP). Therefore, C6S in PEG gels inhibits neurite outgrowth.
Figure 5. Effect of C6S and NGF on cortical neuron outgrowth. Neurons were cultured on gels with and without C6S and/or NGF for 48 h. The average neurite length (A), maximum neurite length (B), and number of neurites (C) were determined for each gel composition. Mean ± SE, *p < 0.05 relative to PEG-BP, #p < 0.05 relative to PEG-BP-C6S, +p < 0.05 relative to PEG-BP-NGF.
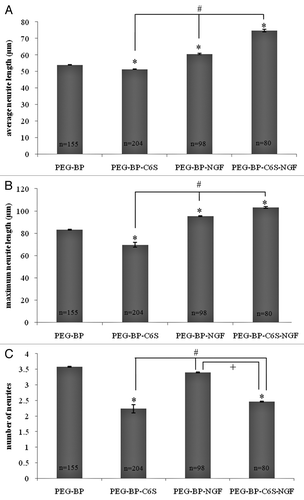
When NGF was incorporated into PEG gels (PEG-BP-NGF), the average and maximum neurite lengths were significantly higher than gels without NGF (PEG-BP and PEG-BP-C6S). In gels with C6S, the neurite length with NGF (PEG-BP-C6S-NGF) was significantly higher than gels without NGF (PEG-BP and PEG-BP-C6S). These results () show that NGF incorporation into PEG gels not only stimulates neurite growth, but also overcomes the inhibitory effects of C6S.
Neurons cultured on PEG-BP-NGF had the same number of neurites as neurons cultured on PEG-BP. Furthermore, neurons cultured on PEG-BP-C6S-NGF had the same number of neurites as neurons cultured on PEG-C6S (). Therefore, NGF had no effect on the number of neurites. The results from cortical neuron culture on PEG gels () showed that C6S decreased neurite outgrowth, while NGF increased neurite length.
Discussion
The PEG-co-peptide polysaccharide biomaterial developed by Seal and PanitchCitation24-Citation26 is a model system to investigate the effects of affinity-based delivery of biological molecules on cortical neuron behavior. In this study, we modified the PEG-co-peptide polysaccharide system by incorporating C6S through C6S-binding peptides conjugated to eight-arm PEG rather than using heparin and heparin-binding peptides as done in the original materials. Rheological results shown in demonstrate that PEG gels that included C6S-binding peptide and/or C6S were weaker than PEG gels without C6S and C6S-binding peptide. The cross-linking reaction of the bi-functional peptides with PEG-maleimide (Mal) is extremely fast. It is possible that C6S and C6S-binding peptides, which were added to the PEG-Mal solution before cross-linking, act as physical barriers between cross-linking sites on different eight-arm PEG molecules, leading to decreased intermolecular cross-linking and weaker gels. It is also possible that after conjugation of two C6S-binding peptides to the star polymer, the efficiency of the cross-linking of the remaining six arms is reduced. Indeed, when C6S and C6S-binding peptide are both incorporated into gels, the PEG gels are the weakest. Further studies are needed to elucidate why cross-linking is suppressed.
The mechanical strength of the gels may affect NGF release from the PEG gels. In the NGF release assay, PEG gels with only C6S and no C6S-binding peptide had the fastest NGF release. Since these gels are weaker than PEG gels without C6S, it is likely that the cross-link density is decreased, thus increasing the molecular weight between cross-links and the average pore size. NGF could have quickly diffused out of the larger pores that are characteristic of lower cross-link density gels. The NGF assay also showed that PEG gels with C6S and C6S-binding peptide released NGF the slowest compared with all other gels. Although the PEG-BP-C6S gel was the most compliant, the binding between NGF, C6S and C6S-binding peptide was strong enough to prevent rapid diffusion from the weaker gel. These results show that C6S and the C6S-binding peptide are both important for slow diffusion of NGF from PEG gels and, possibly, point to a synergistic NGF-binding activity between C6S and the C6S-binding peptide, since NGF release was slowest when gels contained both of these molecules. This hypothesis is supported by other work with C6S gels, where fluorescence recovery after photobleaching studies demonstrated that C6S gels containing this C6S-binding peptide exhibited lower NGF diffusivity when compared with gels with C6S only.Citation30
One of the goals of the current study was to investigate the effects of C6S immobilized in gels on cortical neuron behavior. Previous studies showed that DRG neurite extension is inhibited by chondroitin 4,6-sulfateCitation6 and dermatan sulfateCitation12 immobilized in agarose gels, however, the CS used in these studies was modified for covalent attachment, which may interfere with CS-cell interactions. Therefore, the PEG-peptide polysaccharide system described in the current study is an ideal biomaterial for investigating the effects of unmodified CS in gels. Primary cortical neurons were used in this study to model cellular behavior following injury to the central nervous system.Citation31 Results showed that neurite length and number of neurites were significantly lower in gels with C6S compared with gels without C6S and demonstrate that C6S in PEG gels inhibits cortical neurite outgrowth.
CS can influence cell behavior not only through direct interaction, but also indirectly, through growth factor modulation.Citation19,Citation32 Thus, another goal of this study was to investigate whether the addition of NGF to the system could overcome the inhibitory effects of C6S; this was motivated by studies by Zhou et al. that showed that NGF promotes DRG growth over CSPG-coated slides.Citation33-Citation35 In the current study, the effects of C6S and NGF in gels on cortical neurons were investigated. In PEG gels with only NGF, the average and maximum neurite length was significantly greater than in PEG gels without NGF. This result agrees with previous studies that demonstrate the growth-promoting effects of NGF both in vitro and in vivo.Citation34,Citation35 In PEG gels with C6S and NGF, the neurite length was significantly greater than PEG gels without C6S and NGF. However, the number of neurites in PEG gels with C6S and NGF was significantly less than PEG gels without C6S and NGF. Therefore, NGF can overcome the inhibitory effects of C6S for neurite extension but not for neurite branching and outgrowth, resulting in neurons with fewer and longer neurites. Furthermore, it has been shown that addition of the C6S-binding peptide to matrices of C6S boosts their ability to support DRG growth, possibly through enhanced sequestering of NGF inside the hydrogel and blocking of any potential C6S inhibition.Citation30
In general, C6S inhibits neurite outgrowth and acts as a barrier for axon connections during development and after spinal cord injury. It is hypothesized that C6S provides an inhibitory substrate with which growth-promoting molecules, such as growth factors, can adhere, and provide guidance cues for neurite extension and connection.Citation32 Therefore, the PEG-peptide polysaccharide system that incorporates C6S and NGF through non-covalent interactions is an ideal model for the developing central nervous system.
One of the goals of neural tissue engineering is to promote axonal regeneration for functional recovery. Often, the use of NGF and other growth-promoting molecules causes an uncontrolled increase in neurite outgrowth, which can lead to superfluous connections that cause side effects, such as neuropathic pain.Citation36 Therefore, it is important that for neural tissue engineering, the biomaterial must not only encourage neurite outgrowth but also provide guidance cues for meaningful connections.Citation37 In this study, C6S provides an inhibitory background while NGF promotes neurite outgrowth leading to fewer longer neurites. Future studies will pattern NGF on C6S substrates to guide neurite growth for functional connections.
Conclusions
In this study, the effects of affinity-based delivery of C6S and NGF from gels on cortical neurite behavior were investigated. C6S and NGF were incorporated into PEG gels through C6S-binding peptides. Gels with C6S inhibited neurite outgrowth, while gels with NGF promoted neurite extension. The inhibitory activity of C6S was overcome by NGF, which was slowly released from PEG gels through non-covalent interactions between C6S-binding peptide, C6S and NGF. The affinity-based system developed in this study that incorporates C6S and NGF is an ideal biomaterial for studying neural development and for neural tissue engineering.
Materials and Methods
The C6S-binding peptide was identified through peptide array screening in a previous study.Citation38 The peptides listed in were synthesized through standard Fmoc-solid phase chemistry on a Symphony peptide synthesizer (Protein Technologies, Inc.) at 200 µM with rink amide resin (Anaspec, 20084). Fmoc-protected amino acids were activated by O-(benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate (Synbiosci, REAG2) and added to the growing peptides in 5 M excess. After peptide synthesis, the peptides were cleaved from the resin using a cleave cocktail containing 92.5% triflluoroacetic acid (Acros Organics, 139725000), 2.5% water, 2.5% triisopropylsilane (TCI America, T1533), and 2.5% ethanedithiol (Alfa Aesar, L12865). Peptides were precipitated in 10x excess ice-cold diethyl ether (Mallinckrodt Chemicals, 0848–10) and centrifuged at 5000 rpm for 30 min. The ether was decanted off and the peptide was resolubilized in water before lyophilization.
Table 3. Peptide summary
Peptide purification was performed using reverse phase chromatography with an ÄKTA Explorer system (GE Healthcare) equipped with a C18 column (Grace Vydac; 22 mm internal diameter, 250 mm length, 10–15 µm particle size). After the column was equilibrated with 5 column volumes of water containing 0.1% trifluoroacetic acid, the peptide was loaded onto the column and subjected to an increasing linear gradient from 0 to 60% of acetonitrile (Sigma-Aldrich, 34998) containing 0.1% trifluoroacetic acid over 12 column volumes. The collected peptides were lyophilized, and the mass of each peptide was confirmed with matrix-assisted laser desorption/ionization-time of flight mass spectrometry on a Voyager-DE STR spectrometer (Applied Biosystems).
C6S-binding peptides were conjugated to maleimide-functionalized eight-arm PEG-Mal (MW~40,000 g/mol, Nektar Therapeutics) following a modified version of previous studies.Citation24-Citation27 Peptides were conjugated to PEG-Mal through a Michael-type addition in 1xPBS (pH 7.4, Invitrogen, 14040182) containing 2 mM EDTA (Sigma-Aldrich, E6758). Two arms were conjugated with C6S-binding peptide, while the other six arms were cross-linked with enzymatically degradable bi-functional cross-linking peptide. First, the C6S-binding peptide was conjugated to PEG-Mal by preparing a 2% (w/v) solution of PEG-Mal and C6S-BP at 2-molar excess of eight-arm PEG. The solution was incubated in the dark at room temperature for 1 h. After conjugation, the PEG-co-C6S-BP solution was kept in the dark on ice.
A 2% (w/v) solution of C6S (Sigma-Aldrich, C4384) was added in a 1:4 molar ratio of C6S to PEG-co-peptide. Finally, the bi-functional cross-linking peptide (xlinker) in a 2% (w/v) solution was added in a 3:1 molar ratio of PEG or PEG-BP. The gels were allowed to cross-link for 1 h at room temperature. shows the gel compositions used in this study.
The viscoelastic properties of the hydrogels were measured with a Physica MCR 101 rheometer (Anton Paar) using a parallel-plate geometry with a 20 mm diameter and 100-µm gap. The temperature of the rheometer surface was controlled at 20°C with a built-in Peltier system. To prevent evaporation of the sample, an evaporation-blocking chamber was lowered over the sample. Gels (100 µl) were prepared in triplicate and tested with a frequency and time sweep. The linear range of the viscoelastic response was first measured with a frequency sweep from 0.5–50 rad/s at 0.5% strain. The time sweep was performed at an angular frequency of 10 rad/s and 0.5% strain for 6 min. The complex modulus (G*) of each gel was calculated by averaging the G* over time. To determine the effects of the C6S-binding peptide and C6S on the viscoelastic properties of the material, gels were tested with and without BP and C6S.
The amount of NGF released from gels with and without the C6S-binding peptide and C6S was quantified. Gels (50 µl) were prepared in triplicate in 2 ml siliconized tubes, as previously described, that contained 2 µg/ml NGF (Invitrogen, 13257019). After the gels were cross-linked, 2 ml of 1xPBS was added onto each gel. At each time point, 500 µl of buffer was collected from each tube and immediately replaced with 500 µl of buffer using siliconized pipets and tubes. NGF release was monitored over 48 h, and samples were stored at -20°C. After 2 d, the gels were broken up with a spatula and digested in 10 units/ml collagenase and 0.4 units/ml chondroitinase ABC (Sigma-Aldrich, C3667) for 48 h at 37°C with gentle shaking. The amount of NGF in the collected samples was quantified with a human β-NGF ELISA development kit (Peprotech, 900-K60). The absorbance of each well was measured at 405 nm and 650 nm after 10 min of incubation with ABTS liquid substrate (Sigma-Aldrich, A3219) on a multi-well plate reader (FLUOstar Omega, BMG Labtech). The absorbance at 650 nm was subtracted from the absorbance at 405 nm to determine the relative absorbance of each well, and the amount of NGF was calculated from a standard curve.
To determine the biological activity of C6S and NGF, cortical neurons were cultured on gels with and without C6S and NGF. Gels (20 µl) were prepared according to the previously described protocolCitation39 in silicone inserts (Sigma-Aldrich) placed in chambered glass slides (Nalgene) with two gels per chamber. Silicone inserts were sterilized by sonication with 90% ethanol (VWR Scientific, EM-EX0276) for 20 min. All materials and solutions were filtered (0.2 µm, Millipore) for cell culture.
Cortex tissue (embryonic rat day 18) was purchased from BrainBits. Primary cortical neurons were isolated from E18 cortex tissue following a protocol from BrainBits.Citation40 The cortical tissue was digested in a Hibernate E media solution (BrainBits, HE) containing 2 mg/ml papain (Worthington Biochemical Corporation, LS003126) at 37°C for 30 min. The tissue was then transferred into a 2% (v/v) solution of B27 supplement (Invitrogen, 17504044) in Hibernate media and triturated. The cell suspension was filtered through a 40-µm nylon cell strainer (BD Falcon) and collected. The filtered suspension was centrifuged at 1100 rpm for 1 min. The supernatant was removed and the cell pellet resuspended in 3 ml B27/Neurobasal media (Invitrogen, 21103049) with 0.5 mM glutamine (Invitrogen, 25030149). The viability and density of the cell suspension was determined by mixing 20 μl of Trypan Blue (0.4%, Sigma-Aldrich, T6146) with 20 μl of the cell suspension. Cell density was counted using a hemocytometer. The cell suspension was diluted to a final concentration of 2 x 105 cells/ml.
Supplemented neurobasal media (100 µl) was added onto each gel, then 6.375 µl of the cell suspension was placed on each gel. The cells were incubated for 1 h at 37°C and 5% CO2 before an additional 1 ml of media was added to each chamber (2 gels). Cells were incubated for 48 h before fixation.
After 2 d of culture, the cells were fixed with warm 4% (v/v) paraformaldehyde (Electron Microscopy Sciences, 19200) in 1xPBS for 1 h at room temperature. The cells were permeabilized with 0.1% Triton X-100 (Sigma-Aldrich, T8787) solution in PBS for 2 h. After washing 3x with PBS (20 min incubations), Image-iT™ FX signal enhancer (Invitrogen, I36933) was added to the cells and incubated at room temperature for 2 h. The cells were again washed 3x and blocked overnight with 1% bovine serum albumin (BSA, Sigma-Aldrich, A7906) and 10% goat serum (Invitrogen, 50062Z). After blocking, the cells were washed 6x with 0.1% BSA in PBS then incubated in 5 μg/ml mouse anti-βIII-tubulin (R&D Systems, MAB1195) at room temperature for 2 h and overnight at 4°C. The cells were again washed 3x with 0.1% BSA in PBS and incubated in 2 μg/ml Alexa-488-coupled goat anti-mouse F(ab)’2-fragment secondary antibody (Invitrogen, A11029) for 2 h at room temperature in the dark. Finally, the cells were washed 3x with 0.1% BSA in PBS before visualization.
Images were captured using a Leica DMIRB (Leica Microsystems) epifluorescence microscope. A USH-102DH-100W ultra-high-pressure mercury lamp (USHIO America, Inc.) was used as the excitatory light source. Images were viewed in the blue excitation range (filter set I3- excitation filter BP450–490 nm and emission filter BP515 nm) with a 20 × objective. The images were analyzed with a custom Matlab program that measured the distance of each neurite from the center of the cell body. Approximately 100–200 neurons were analyzed for each treatment.
Statistical analysis was performed with Minitab 15 (Minitab) and SPSS 16 (SPSS). A one-way ANOVA and Tukey’s post-hoc test (α = 0.05) was performed to determine statistical significance (p < 0.05) of the G* of the different gels for the time sweep. Repeated measures of two-factor ANOVA (α = 0.05) were performed to determine statistical significance (p < 0.05) of the G* of the different gels for the frequency sweep and of the NGF release of the different gels. A Tukey’s post-hoc test (α = 0.05) was performed to determine significance of different gels at 10 rad/s for rheology and at 0 to 48 h for the NGF release assay. To determine differences in cortical neuron outgrowth, a Kruskal-Wallis with a Mann-Whitney post-hoc test (α = 0.05) was performed.
| Abbreviations: | ||
| ANOVA | = | analysis of variance |
| BP | = | binding peptide |
| BSA | = | bovine serum albumin |
| C6S | = | chondroitin-6-sulfate |
| CS | = | chondroitin sulfate |
| CSPG | = | chondroitin sulfate proteoglycan |
| DRG | = | dorsal root ganglion |
| EDTA | = | ethylenediaminetetraacetic acid |
| GAG | = | glycosaminoglycan |
| NGF | = | nerve growth factor |
| PEG | = | poly(ethylene glycol) |
| Mal | = | maleimide |
| PBS | = | phosphate buffered saline |
| SE | = | standard error |
Acknowledgments
This work was funded in part though grant 0017 from the Arizona Biomedical Research Commission.
References
- Sugahara K, Mikami T. Chondroitin/dermatan sulfate in the central nervous system. Curr Opin Struct Biol 2007; 17:536 - 45; http://dx.doi.org/10.1016/j.sbi.2007.08.015; PMID: 17928217
- Kwok JCF, Afshari F, Garcia-Alias G, Fawcett J. Proteoglycans in the central nervous system: Plasticity, regeneration and their stimulation with chondroitinase ABC. Restor Neurol Neurosci 2008; 26:131 - 45; PMID: 18820407
- Mizuguchi S, Uyama T, Kitagawa H, Nomura KH, Dejima K, Gengyo-Ando K, et al. Chondroitin proteoglycans are involved in cell division of Caenorhabditis elegans. Nature 2003; 423:443 - 8; http://dx.doi.org/10.1038/nature01635; PMID: 12761550
- Gama CI, Tully SE, Sotogaku N, Clark PM, Rawat M, Vaidehi N, et al. Sulfation patterns of glycosaminoglycans encode molecular recognition and activity. Nat Chem Biol 2006; 2:467 - 73; http://dx.doi.org/10.1038/nchembio810; PMID: 16878128
- Properzi F, Carulli D, Asher RA, Muir E, Camargo LM, van Kuppevelt TH, et al. Chondroitin 6-sulphate synthesis is up-regulated in injured CNS, induced by injury-related cytokines and enhanced in axon-growth inhibitory glia. Eur J Neurosci 2005; 21:378 - 90; http://dx.doi.org/10.1111/j.1460-9568.2005.03876.x; PMID: 15673437
- Gilbert RJ, McKeon RJ, Darr A, Calabro A, Hascall VC, Bellamkonda RV. CS-4,6 is differentially upregulated in glial scar and is a potent inhibitor of neurite extension. Mol Cell Neurosci 2005; 29:545 - 58; http://dx.doi.org/10.1016/j.mcn.2005.04.006; PMID: 15936953
- Clement AM, Sugahara K, Faissner A. Chondroitin sulfate E promotes neurite outgrowth of rat embryonic day 18 hippocampal neurons. Neurosci Lett 1999; 269:125 - 8; http://dx.doi.org/10.1016/S0304-3940(99)00432-2; PMID: 10454148
- Fernaud-Espinosa I, Nieto-Sampedro M, Bovolenta P. Differential effects of glycosaminoglycans on neurite outgrowth from hippocampal and thalamic neurones. J Cell Sci 1994; 107:1437 - 48; PMID: 7962187
- Tully SE, Mabon R, Gama CI, Tsai SM, Liu X, Hsieh-Wilson LC. A chondroitin sulfate small molecule that stimulates neuronal growth. J Am Chem Soc 2004; 126:7736 - 7; http://dx.doi.org/10.1021/ja0484045; PMID: 15212495
- Snow DM, Brown EM, Letourneau PC. Growth cone behavior in the presence of soluble chondroitin sulfate proteoglycan (CSPG), compared to behavior on CSPG bound to laminin or fibronectin. Int J Dev Neurosci 1996; 14:331 - 49; http://dx.doi.org/10.1016/0736-5748(96)00017-2; PMID: 8842808
- Dillon GP, Yu XJ, Bellamkonda RV. The polarity and magnitude of ambient charge influences three-dimensional neurite extension from DRGs. J Biomed Mater Res 2000; 51:510 - 9; http://dx.doi.org/10.1002/1097-4636(20000905)51:3<510::AID-JBM28>3.0.CO;2-G; PMID: 10880096
- Yu X, Bellamkonda RV. Dorsal root ganglia neurite extension is inhibited by mechanical and chondroitin sulfate-rich interfaces. J Neurosci Res 2001; 66:303 - 10; http://dx.doi.org/10.1002/jnr.1225; PMID: 11592128
- Hwang NS, Varghese S, Lee HJ, Theprungsirikul P, Canver A, Sharma B, et al. Response of zonal chondrocytes to extracellular matrix-hydrogels. FEBS Lett 2007; 581:4172 - 8; http://dx.doi.org/10.1016/j.febslet.2007.07.049; PMID: 17692846
- Varghese S, Hwang NS, Canver AC, Theprungsirikul P, Lin DW, Elisseeff J. Chondroitin sulfate based niches for chondrogenic differentiation of mesenchymal stem cells. Matrix Biol 2008; 27:12 - 21; http://dx.doi.org/10.1016/j.matbio.2007.07.002; PMID: 17689060
- Liu Y, Cai SS, Shu XZ, Shelby J, Prestwich GD. Release of basic fibroblast growth factor from a cross-linked glycosaminoglycan hydrogel promotes wound healing. Wound Repair Regen 2007; 15:245 - 51; http://dx.doi.org/10.1111/j.1524-475X.2007.00211.x; PMID: 17352757
- Piai JF, Rubira AF, Muniz EC. Self-assembly of a swollen chitosan/chondroitin sulfate hydrogel by outward diffusion of the chondroitin sulfate chains. Acta Biomater 2009; 5:2601 - 9; http://dx.doi.org/10.1016/j.actbio.2009.03.035; PMID: 19394902
- Sakiyama-Elbert SE, Hubbell JA. Controlled release of nerve growth factor from a heparin-containing fibrin-based cell ingrowth matrix. J Control Release 2000; 69:149 - 58; http://dx.doi.org/10.1016/S0168-3659(00)00296-0; PMID: 11018553
- Sakiyama-Elbert SE, Hubbell JA. Development of fibrin derivatives for controlled release of heparin-binding growth factors. J Control Release 2000; 65:389 - 402; http://dx.doi.org/10.1016/S0168-3659(99)00221-7; PMID: 10699297
- Raman R, Sasisekharan V, Sasisekharan R. Structural insights into biological roles of protein-glycosaminoglycan interactions. Chem Biol 2005; 12:267 - 77; http://dx.doi.org/10.1016/j.chembiol.2004.11.020; PMID: 15797210
- Willerth SM, Johnson PJ, Maxwell DJ, Parsons SR, Doukas ME, Sakiyama-Elbert SE. Rationally designed peptides for controlled release of nerve growth factor from fibrin matrices. J Biomed Mater Res A 2007; 80:13 - 23; http://dx.doi.org/10.1002/jbm.a.30844; PMID: 16958043
- Wood MD, Borschel GH, Sakiyama-Elbert SE. Controlled release of glial-derived neurotrophic factor from fibrin matrices containing an affinity-based delivery system. J Biomed Mater Res A 2009; 89:909 - 18; http://dx.doi.org/10.1002/jbm.a.32043; PMID: 18465825
- Maxwell DJ, Hicks BC, Parsons S, Sakiyama-Elbert SE. Development of rationally designed affinity-based drug delivery systems. Acta Biomater 2005; 1:101 - 13; http://dx.doi.org/10.1016/j.actbio.2004.09.002; PMID: 16701784
- Wood MD, Moore AM, Hunter DA, Tuffaha S, Borschel GH, Mackinnon SE, et al. Affinity-based release of glial-derived neurotrophic factor from fibrin matrices enhances sciatic nerve regeneration. Acta Biomater 2009; 5:959 - 68; http://dx.doi.org/10.1016/j.actbio.2008.11.008; PMID: 19103514
- Seal BL, Panitch A. Physical polymer matrices based on affinity interactions between peptides and polysaccharides. Biomacromolecules 2003; 4:1572 - 82; http://dx.doi.org/10.1021/bm0342032; PMID: 14606882
- Seal BL, Panitch A. Physical matrices stabilized by enzymatically sensitive covalent cross-links. Acta Biomater 2006; 2:241 - 51; http://dx.doi.org/10.1016/j.actbio.2005.12.008; PMID: 16701884
- Seal BL, Panitch A. Viscoelastic behavior of environmentally sensitive biomimetic polymer matrices. Macromolecules 2006; 39:2268 - 74; http://dx.doi.org/10.1021/ma0524528
- Jeong KJ, Panitch A. Interplay between covalent and physical interactions within environment sensitive hydrogels. Biomacromolecules 2009; 10:1090 - 9; http://dx.doi.org/10.1021/bm801270k; PMID: 19301930
- Conovaloff A, Panitch A. Characterization of a chondroitin sulfate hydrogel for nerve root regeneration. J Neural Eng 2011; 8:056003; http://dx.doi.org/10.1088/1741-2560/8/5/056003; PMID: 21804177
- Butterfield KC, Conovaloff A, Caplan M, Panitch A. Chondroitin sulfate-binding peptides block chondroitin 6-sulfate inhibition of cortical neurite growth. Neurosci Lett 2010; 478:82 - 7; http://dx.doi.org/10.1016/j.neulet.2010.04.070; PMID: 20450957
- Conovaloff A, Beier B, Irazoqui P, Panitch A. Effects of a synthetic bioactive peptide on neurite growth and nerve growth factor release in chondroitin sulfate hydrogels. Biomatter 2011; In press
- Hains BC, Black JA, Waxman SG. Primary cortical motor neurons undergo apoptosis after axotomizing spinal cord injury. J Comp Neurol 2003; 462:328 - 41; http://dx.doi.org/10.1002/cne.10733; PMID: 12794736
- Carulli D, Laabs T, Geller HM, Fawcett JW. Chondroitin sulfate proteoglycans in neural development and regeneration. Curr Opin Neurobiol 2005; 15:116 - 20; http://dx.doi.org/10.1016/j.conb.2005.03.018; PMID: 15721753
- Zhou FQ, Walzer M, Wu YH, Zhou J, Dedhar S, Snider WD. Neurotrophins support regenerative axon assembly over CSPGs by an ECM-integrin-independent mechanism. J Cell Sci 2006; 119:2787 - 96; http://dx.doi.org/10.1242/jcs.03016; PMID: 16772333
- Bloch J, Fine EG, Bouche N, Zurn AD, Aebischer P. Nerve growth factor- and neurotrophin-3-releasing guidance channels promote regeneration of the transected rat dorsal root. Exp Neurol 2001; 172:425 - 32; http://dx.doi.org/10.1006/exnr.2001.7778; PMID: 11716566
- Colangelo AM, Finotti N, Ceriani M, Alberghina L, Martegani E, Aloe L, et al. Recombinant human nerve growth factor with a marked activity in vitro and in vivo. Proc Natl Acad Sci USA 2005; 102:18658 - 63; http://dx.doi.org/10.1073/pnas.0508734102; PMID: 16339317
- Romero MI, Rangappa N, Garry MG, Smith GM. Functional regeneration of chronically injured sensory afferents into adult spinal cord after neurotrophin gene therapy. J Neurosci 2001; 21:8408 - 16; PMID: 11606629
- Yu LMY, Leipzig ND, Shoichet MS. Promoting neuron adhesion and growth. Mater Today 2008; 11:36 - 43; http://dx.doi.org/10.1016/S1369-7021(08)70088-9
- Butterfield KC, Caplan M, Panitch A. Identification and sequence composition characterization of chondroitin sulfate-binding peptides through peptide array screening. Biochemistry 2010; 49:1549 - 55; http://dx.doi.org/10.1021/bi9021044; PMID: 20095636
- Eng D, Caplan M, Preul M, Panitch A. Hyaluronan scaffolds: A balance between backbone functionalization and bioactivity. Acta Biomater 2010; 6:2407 - 14; http://dx.doi.org/10.1016/j.actbio.2009.12.049; PMID: 20051273
- Brewer GJ. Serum-free B27/Neurobasal medium supports differentiated growth of neurons from the striatum, substantia-nigra, septum, cerebral-cortex, cerebellum, and dentate gyrus. J Neurosci Res 1995; 42:674 - 83; http://dx.doi.org/10.1002/jnr.490420510; PMID: 8600300