Abstract
In this study, the development and characterization of novel polymer blends based on chitosan-poly (vinyl alcohol) and physically cross-linked by freeze-thaw method for possible use in a variety of biomedical application is reported. The present investigation deals with designing savlon-loaded blend hydrogels (coined as cryogels) of poly (vinyl alcohol) (PVA) and chitosan by repeated freeze-thaw method and their characterization by SEM and FTIR techniques. The FTIR spectra clearly reveal that savlon-loaded chitosan and PVA blends are bonded together through hydrogen bonding. The SEM analysis suggests that cryogels show a well-defined porous morphology. The prepared cryogels were also investigated for swelling and deswelling behaviors. The results reveal that both the swelling and deswelling behaviors greatly depend on factors like chemical composition of the cryogels, number of freeze-thaw cycles, pH and temperature of the swelling bath. The savlon-loaded blends were also investigated for their in vitro blood compatibility and antibacterial activity.
Introduction
Wound healing is a specific biological regeneration process of damaged and/or lost tissues.Citation1 An appropriate wound dressing should be able to enhance the healing process considerably by mediating at the right stage of or providing excellent conditions for wound healing.Citation2 In general, an effectual wound dressing should maintain a moist environment upon absorption of the wound exudates, protect the wound from secondary infection, reduce necrosis of the wound bed, provide adequate gaseous exchange, regulate and/or mediate the release of certain growth factors and cytokines and also be elastic biocompatible with tissues and blood; it should be non-toxic and non-antigenic.Citation3,Citation4
Based on these requirements, biocompatible polymeric hydrogels emerge as promising materials for use as wound dressings, since they can be tailor-made to meet specific needs.Citation5 Such needs, in addition to the general requirements for an effectual wound dressing, include non-irritating and non-adhering properties, immediate pain relief, ease of handling and replacement without compromising patients’ comfort, transparency to allow easy monitoring of the wound bed and facilitation of the migration and mitosis of epithelial cells. By definition, hydrogels are three-dimensional, hydrophilic, water-insoluble polymeric networks.Citation6-Citation10 The internal networks may result from physical and/or chemical domains that retain their integrity, either in whole or in part, when surrounded by a large amount of water molecules. The functions of hydrogels in biomedical applications, including as wound dressings, originate due to their ability to imbibe a large quantity of water, which, consequently, imparts unusual biophysical properties to the polymer.
PVA and chitosan are synthetic and natural polymers, respectively, and are able to form physically cross-linked hydrogels by a variety of techniques, such as chemical cross-linkingCitation11 or the freeze-thaw method.Citation12 In the present study, we adopted a cryogenic approach, blending chitosan with PVA at different ratios, followed by successive freezing-thawing operations. The cryogenic method is performed over conventional chemical cross-linking, as in the former process, but no chemical toxicity remains with the end product.
In chemical cross-linking, glutaraldehye has been broadly used as an active chemical cross-linker of PVA and chitosan due to its ability to form the formation of intra-interchain covalent bonding. Nevertheless, this synthetic cross-linker has been reported as highly cytotoxic, which may impair the biocompatibility of the cross-linked biomaterials. These disadvantages can, however, be minimized by ensuring that all aldehyde functional groups have actually cross-linked with the polymeric network, or they can be effectively blocked with molecules, such as amino acids and proteins which are widely presented in living organism serum.Citation13 On the other hand, the physical cross-linking phenomenon (freeze-thaw method) is based on the existence of regular pendant hydroxyl groups on PVA that are able to form crystallites by strong inter-chain hydrogen bonding.Citation14 This method produces stable gels that are cross-linked by the presence of crystalline regions.Citation15
Advantages of physically cross-linked hydrogels are their non-toxicity, non-carcinogenicity, high elasticity and the good biocompatibility of the resulting polymer.Citation16
Chitosan is a carbohydrate biopolymer derived from deacetylation of chitin, the main component of crustacean (e.g., shrimp, crab, lobster) exoskeleton.Citation17 It is a medically important biopolymer due to its biological properties, such as its antimicrobial, hemostatic and antitumor activities, its acceleration of the wound healing process, use in tissue engineering scaffolds and promise for drug delivery.Citation18 It is also biodegradable, hydrophilic and biocompatible, with low toxicity to mammalian cells.Citation19
Chitosan has received great attention for medical and pharmaceutical applications due to its beneficial intrinsic properties. It is one of the natural polymers that has shown a high potential in wound healing applications.Citation20 It is well known for being able to accelerate the healing of wounds in humans, and it has also been documented that chitosan confers considerable antibacterial activity against a broad spectrum of bacteria.Citation21
PVA is a unique material even in atatic form and is semi-crystalline despite its lack of stereo regularity.Citation22 In aqueous solutions with a polymer concentration of more than 1%, entangled aggregates of hydrogen-bonded PVA molecules are formed. This is a consequence of the formation of crystalline regions.Citation23 It is often used in biomedical applications. It is a water-soluble synthetic polymer with excellent film-forming, emulsifying and adhesive properties.Citation24
PVA hydrogels prepared with freeze-thaw techniques have great potential for biomedical applications. This is due to a high swelling capability, often coupled with relatively good mechanical properties and biocompatibility.Citation25 Biomedical applications include soft contact lenses, implants, artificial organs and drug delivery devices. This is because of the characteristics of PVA, such as biodegradability,Citation26 inherent non-toxicity, non-carcinogenicity, good biocompatibility and desirable physical properties, such as its rubbery or elastic nature.Citation27,Citation28
Savlon is a well-known antiseptic liquid. Its aqueous solution contains chlorohexadiene gluconate and cetrimede as active ingredients and n-propyl alcohol and benzyl benzoate as preservatives. It is a clear, orange-colored liquid with a pine-like odor. The present study aims to design a wound dressing patch of PVA and chitosan prepared by repeated freeze-thaw method and to study its water sorption and antibacterial behavior.
Results
Characterization
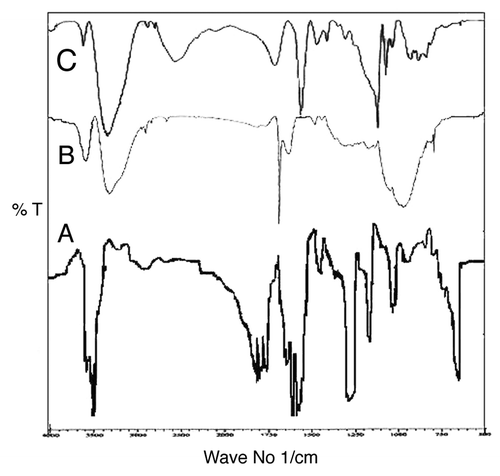
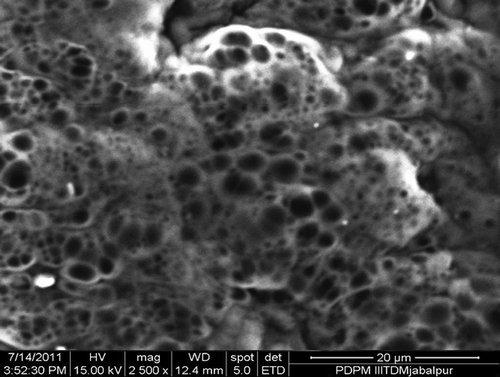
In order to confirm the structure of prepared savlon-loaded hydrogels and to study the morphology of the prepared polymer materials, FTIR spectra and ESEM analysis were performed, respectively. The obtained spectra of PVA, savlon and savlon-absorbed hydrogels are shown in , respectively, whereas the microscopic image of the hydrogel is shown in .
Effect of PVA/chitosan
The chemical composition of the cryogel matrix has a great impact on the final performance of the material. In the present study, two polymers, namely, PVA and chitosan, were used. The former is a synthetic and hydrophilic polymer, whereas the latter is cationic and hydrophilic in nature. For studying the effect of varying composition of the matrix on water imbibition properties of the hydrogel, the PVA/chitosan wt. fraction was varied from 2.9 to 12.1, and the results are presented in . It is clear from the observed findings that the capacity of hydrogels to retain water increases up to 4.0 wt. fraction and decreases upon further increasing the wt. fraction of the two polymers.
Effect of savlon
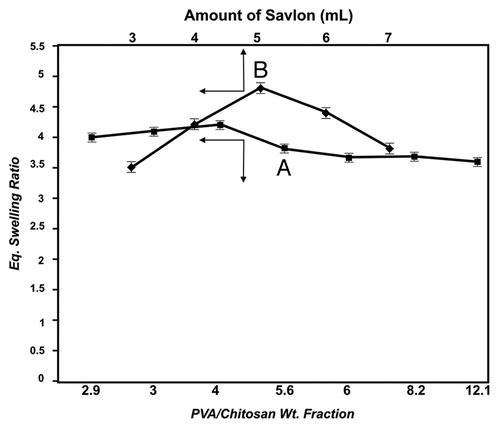
Since savlon has been used as disinfectant in the present study, its influence on the swelling property of the cryogel becomes a factor of much significance. In order to evaluate its effect, the amount of savlon present in the cryogel was varied from 3–7 mL in the reaction mixture. The equilibrium swelling results are shown in and indicate that the water sorption capacity gradually increases with 3–5mL of added savlon, while beyond 5mL of savlon, a fall in water sorption capacity is noted.
Effect of freeze-thaw cycles
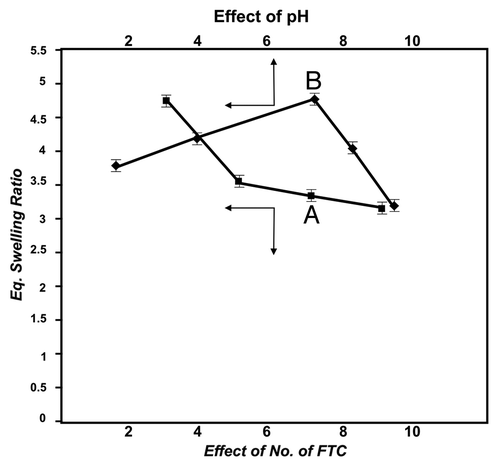
The internal structure of a polymer matrix greatly depends on the number of cross-linkages present in the network. In the present study, since the cross-linking was achieved through freeze-thaw cycles, the influence of these cycles on the water sorption capacity of hydrogels was investigated by increasing the number of freeze-thaw cycles from 3 to 9 and monitoring the change in the equilibrium swelling ratio. The results are presented in and indicate a constant drop in swelling ratio when the number of freeze-thaw cycles is increased in the studied range.
Effect of pH
pH of the swelling medium is an important experimental parameter that directly affects water intake capacity of the hydrogel. In this study, the pH of the swelling bath was varied from 4.0–9.2, and equilibrium swelling was noticed. The results presented in indicate that when the pH of the medium increases from 1.8–7.4, the water sorption capacity constantly increases, whereas beyond 7.4 pH, a decrease in water retention property is observed.
Effect of temperature
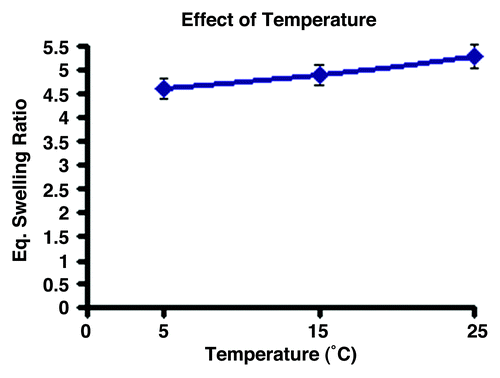
Temperature is another important parameter that affects the water sorption capacity of polymer networks. The underlying reason for this fact is that a change in temperature directly affects mobility of water molecules, movement of polymeric chains of the network and interaction between polymer network and water molecules. In this study, the effect of temperature on equilibrium swelling ratio was also studied by increasing the temperature from 5–25°C. The observed swelling results are shown in , which reveals that when the temperature is raised in the studied range, a increase in water sorption capacity is observed.
Effect of simulated biofluids
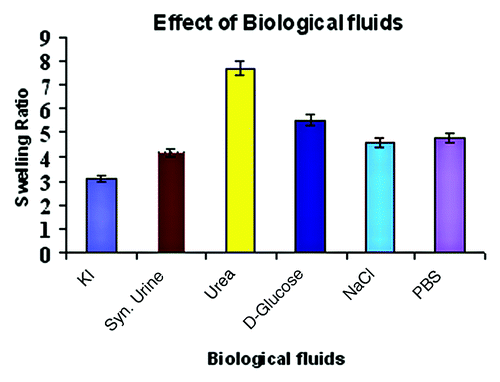
The nature of a swelling medium is a significant factor that brings about a change in water sorption behavior of the polymer network when it’s allowed to swell. It is theoretically as well as practically known that the extent of swelling of polymer gels in a medium is due to the compacting effects of osmotic pressure produced across the gel and the swelling medium and the restoring tendency of elastic chains of the network. When inorganic salt or organic molecules are present in the swelling medium, the osmotic pressures as well as restoring capacity of network chains are affected. In the present investigation, the effect of the nature of the swelling medium has also been studied by allowing prepared cryogel to swell in the presence of compounds like D-glucose (5%w/v), KI (15%w/v), simulated biological fluids, like saline water (0.9%w/v), and artificial urine. The swelling results are shown in , which indicates that sorption is significantly affected in these swelling media.
Deswelling studies
For a polymer material to be employed as hydrogel dressing or wound dressing material, it is important to evaluate how long the polymer matrix can hold water molecules within the matrix. This property is quite important and greatly depends on the chemical composition of the cryogels. In the present study, therefore, the progress of the deswelling process was monitored for cryogel of different compositions, as discussed in the following paragraphs.
When the wt. fraction of PVA/chitosan is varied in the feed mixture from 2.9–2.1, a continuous fall in % deswelling is noticed, as depicted in .
Figure 7. (A) Effect of wt. fraction of PVA/chitosan on deswelling behaviors of the cryogels. (B) Influence of amount of savlon (mL) on the deswelling of cryogel.
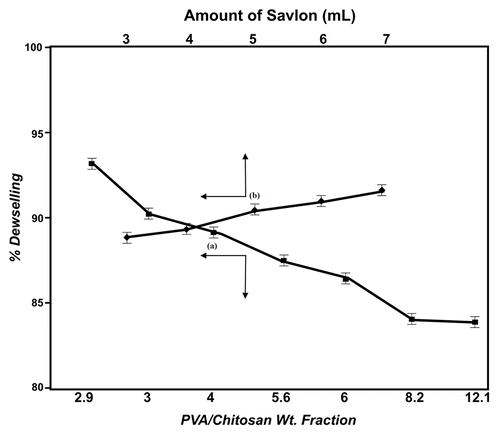
In a similar way, the quantity of savlon present in the hydrogel matrix is also expected to affect the swelling nature of the hydrogel. This has been studied by adding savlon in the range 3 to 7mL to the feed composition. The results obtained are depicted in , which shows that the percent deswelling constantly increases with increasing amount of savlon. In this way, the cryogel shows the lowest equilibrium deswelling. when 3mL of savlon is presented in the cryogel.
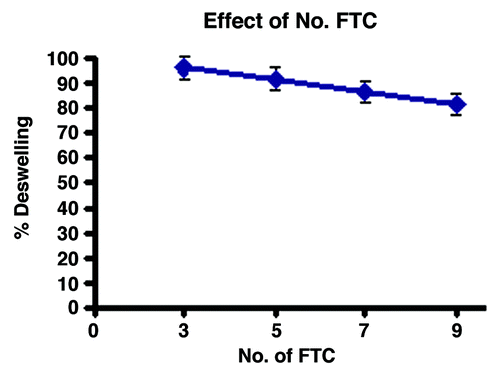
In order to study the effect of freeze-thaw cycles on deswelling behavior of the gel, the cryogels were prepared with an increasing number of freeze-thaw cycles, ranging from 3 to 9. The results are shown in , which indicate that with increasing number of freeze-thaw cycles, the % deswelling constantly decreases.
In vitro blood compatibility
A biomaterial may be defined as a synthetic or natural material that is put in contact with the flowing blood or within the body for a specific purpose and for a predetermined duration. It is well known that every material cannot be considered for use as a biomaterial, since as soon as the body recognizes a foreign material inside it, several undesirable and adverse consequences may occur, thus leading to ultimate failure of the device. It is therefore essential that the biomaterial must fulfill certain requirements in order to deserve consideration for subsequent use as biomaterials. Extensive investigations have been performed to judge the biocompatibility of different materials, and it is now recognized that chemical composition and surface topology are important factors in determining the suitability of the materials for biomedical purposes. As a preliminary investigation, the in vitro biocompatibility is often determined on the basis of certain tests, such as blood clot formation and hemolysis assessment. In the present investigation, these two tests were performed, and results are summarized in and discussed below.
Table 1. Data showing the biocompatibility parameters with varying composition of cryogels
The data summarized in clearly indicate that no detectable amount of blood clots was found on the cryogel surfaces, which suggests that the prepared cryogels’ surfaces are antithrombogenic in nature.
In a similar way, the cryogels of different compositions were analyzed for hemolysis assessment, and the obtained data are shown in . It may be concluded from the data that when the wt. fraction of PVA/chitosan increases from 2.9–12.1, the % hemolysis constantly increases. When the amount of savlon in the cryogel increases form 3–5 mL, a decrease is observed in % hemolysis, while beyond 5 mL of savlon content the % hemolysis increases.
Antibacterial assay
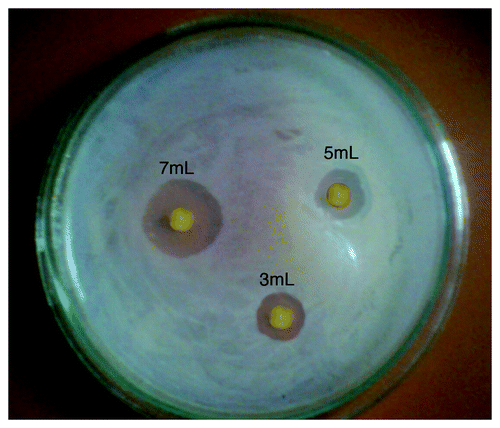
In the current study, the Disc Diffusion method was used to evaluate the bactericidal effect of savlon-loaded cryogels. The antibacterial activity of savlon-loaded cryogels is shown in , which indicates that disinfectants have broad activity against E.coli, and the zone of inhibition increases with increasing amount of disinfectant loaded on the cryogels in the range of 3 to 7 mL.
Effect of FTC on morphology
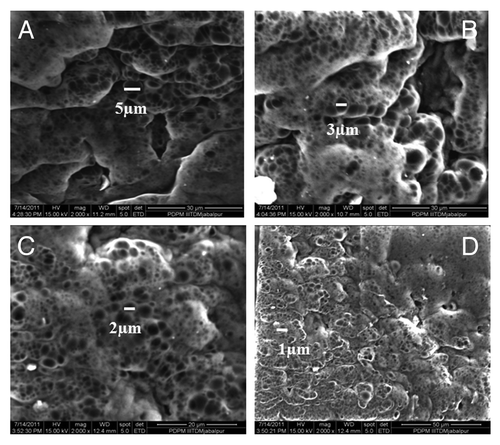
The morphology of the physically cross-linked network greatly depends on the number of FTC, which significantly alters the dimensions of a porous network. In the present study, the number of FTC was varied in the range of 3 to 9, and change in the morphology of the cryogel was noted by recording the ESEM images of the network. The results are depicted in , which reveals that the pore sizes decreases with increasing number of FTC.
Discussion
The FTIR spectra provide information about the functional groups of constituent polymers present in the cryogel. The spectra shown in indicate the presence of poly(vinyl) alcohol and savlon, as evident from the peaks observed at 3449 cm−1 and 3404 cm−1 respectively.Citation29 The spectra () clearly mark a peak around 3683 cm−1, which is due to the overlapping peak of OH of PVA and NH2 group of chitosan.Citation30 The IR spectra () also shows sharp peak around 1521 cm−1 (N-H bending) due to presence of CHX of savlon group.Citation31 It is clear from the above discussion on the peaks that constituent polymers PVA and chitosan and savlon are present in the prepared cryogels.
The effect of the PVA/chitosan wt. fraction on the extent of water sorption indicates that the swelling ratio increases when the PVA/chitosan wt. fraction increases from 2.9 to 4.0, and thereafter the equilibrium swelling constantly decreases. Thus an optimum swelling is reached at a PVA/chitosan wt. fraction of 4.0. The results may be explained as below.
The water sorption capacity of cryogels is also affected by the changing wt. fraction of PVA/chitosan contained in the cryogel. The results clearly show that water intake capacity constantly increases when the wt. fraction increases from 2.9–4.0, while further increasing the wt. fraction results in a fall in the water sorption capacity. This clearly indicates that when the wt. fraction of PVA/chitosan is 4.0, the swelling becomes optimum. The observed findings may be explained as below.
Since both the constituents polymers, that is PVA and chitosan, are hydrophilic in nature, their increasing wt. fraction results in enhanced hydrophilicity of the matrix, which results in an increased water sorption capacity. However, beyond 4.0 wt. fraction of PVA, the water sorption capacity falls, which may be explained by the fact that when the PVA content is high and the resulting cryogel is enriched in crystalline region of PVA, this accounts for lower water sorption tendency of the cryogel. Similarly, when the wt. fraction of chitosan decreases from 12.1 to 4.0, the water intake capacity of the cryogels increases up to 4.0 wt. fraction, whereas a decrease in equilibrium swelling is noticed thereafter. The results may be attributed to the fact that chitosan is a cationic biopolymer, and its increasing content in the cryogel results in loosening of the network chains due to existing repulsion between the cationic chains of chitosan. However, when the chitosan wt. fraction increases beyond 4.0, the network becomes densely crowed with the polymer chains, and water sorption capacity of the gel decreases.
The presence of disinfectant savlon also affects the overall swelling ratio of the cryogel. It is observed that when the savlon content increases from 3 to 5 mL in the cryogel, the swelling ratio also increases, whereas beyond 5mL of added savlon, a decrease in swelling ratio is observed. The results may be attributed to the fact that savlon contains cetrimide, which is ionic in nature, and when the savlon content increases, the concentration of ionic species also increase inside the cryogel matrix. The increase in ionic content in the cryogel results in an increased osmotic pressure of the network and enhances relaxation of network chains. Both of these factors tend to increase the swelling ratio of the cryogel. However, the decrease in swelling ratio beyond 5 mL of added savlon lowers the water sorption capacity of the cryogel, which may be explained due to binding of savlon components to the chitosan and PVA chains of the gel, thus decreasing the chain mobility. This clearly results in a lower water sorption capacity.
When the number of FTC increases from 3 to 9, a constant fall in equilibrium swelling ratio is noticed. The reason behind the observed decrease in swelling ratio is that with increasing number of FTC, the gel acquires increasing crystallinity of the network, which consequently lowers the movements of savlon-loaded network chains. Another reason for the observed lower value of water sorption capacity of the cryogel may be that an increasing number of freeze-thaw cycles results in greater intermolecular forces between the PVA and chitosan chains. The enhanced binding forces between the network chains reduce pore sizes of the network, and, therefore, the water sorption capacity decreases.Citation32 The proposed explanation for the observed shrinking of pore sizes of the network is further evident from the observed ESEM images of the cryogel shown in . The ESEM images clearly show that with increasing number of freeze-thaw cycles, the pore size of the cryogels surfaces also decreases. Decrease in pore sizes with increasing number of FTC is a widely reported result.
pH is an important parameter, and it significantly affects water sorption properties of the polymer matrix, especially when the polymer components of the matrix bear a charge. In the present study, it is found that when pH of the swelling medium increases from 4.0–7.4 pH, there occurs an increase in water sorption capacity of the cryogels, whereas beyond pH 7.4, a decrease in equilibrium swelling ratio is noticed. The observed finding may be attributed to the reason that both chitosan macromolecules and cetrimide are positively charged species, and when the savlon-loaded cryogel is emerged in swelling medium of low pH, H+ ions defuse into the cryogels network and produce repulsion inside the bulk of the matrix. The repulsive forces result in a relaxation of cryogel chains, which allows a greater number of water molecule to enter into the gel. This obviously increases the water intake capacity of the cryogels. However, beyond pH 7.4, the observed decrease in equilibrium swelling may be attributed to the fact that the hydroxyl ions from the alkaline swelling medium enter the gel and neutralize the H+ ions of chitosan chains. In this way, the repulsive forces inside the matrix are minimized, which also results in a decrease in water sorption capacity of the cryogel.
An increase in temperature often results in greater water sorption by the hydrogel. In the present study, the increase in temperature also brings about an increase in the swelling ratio, which could be attributed to the fact that a rise in temperature of the swelling medium may result in faster movements of water molecules into the gel as well as greater relaxation of polymer chains. All these factors result in an increase in water sorption capacity of the cryogel.Citation33 In simulated physiological fluids, a lower swelling ratio was obtained, which could be attributed to the fact that in physiological fluids, which normally contain salt ions and organic molecules, result in a decrease in osmotic pressure up the cryogel, thus the decreasing the swelling ratio.Citation34
The slow deswelling properties of the cryogels make them a suitable candidate for wound healing and burn dressing applications. It is the chemical composition of the matrix that greatly affects deswelling properties in a swollen hydrogel. In the present study, it has been found that the % deswelling continuously decreases with increasing PVA/chitosan wt. fraction of the cryogel. These results may be attributed to the fact that increasing the amount of PVA in the cryogel makes the polymer matrix more hydrophilic in nature, and therefore water molecules are tightly held by PVA chains, and, as a result, the matrix shows a decreasing tendency to lose water. This eventually results in a decreased deswelling nature of the cryogel.
Savlon content in the loaded cryogel also exerts a great impact on % deswelling of the cryogel. It is observed that on increasing the savlon in the feed mixture from 3–7 mL, there is a slight increase in deswelling properties of the gel, which could be attributed to the fact that increasing the amount of savlon in the cryogel produces greater hydrophobicity in the matrix, leading to rapid loss of water molecules from the gel. Another reason for the observed findings may be that both chitosan and cetrimide molecules present in the savlon are positively charged species, and increasing savlon may cause enhanced repulsion within the deswelling matrix. This consequently produces frequent relaxation of polymer chains, thus allowing easy escape of water molecules from the cryogels and resulting in an increase in deswelling of the material.
Another important parameter that greatly affects internal structure of the hydrogel matrix is the number of freeze-thaw cycles through which the cryogel has been prepared. It is observed that the % deswelling of the matrix decreases with increasing numbers of FTC in the range 3 to 9. The reason for the observed decrease in % deswelling is that with increasing number of freeze-thaw cycles, the pore sizes of the network subsequently decrease, permitting a smaller number of water molecules to escape. This clearly explains the observed decreasing % deswelling of the cryogel.
The chemical composition of a hydrogel is the key factor affecting blood compatibility of the proposed biomaterials. In this study, the blood compatibility of the cryogels has been found to be fairly good. The possible explanation for the observed unusual fair blood compatibility may be the hydrophilic and biocompatible nature of the constituent PVA and chitosan. Alternatively, since only physical cross-linking is involved in the preparation of cryogels in the present study and no chemical cross-linking agent was used, the prepared cryogels obviously show excellent antithrombogenic property.
The % hemolysis data indicate that it varies in the range 22.5–44.3% only, which implies for a fair biocompatibility of the matrix.
The prepared cryogels show excellent antibacterial properties, as evident from the photograph shown in the results section. The results suggest that an increasing amount of savlon shows increasing effective antibacterial property against E.coli, and the largest zone of inhibition is seen with 7 mL of savlon added.
The increasing number of freeze-thaw cycles significantly reduces the pore sizes of the cryogels. The reason for the observed shrinkage in pore size may be that increasing the number of freeze-thaw cycles results in building of larger areas of crystalline regions in the cryogel and enhanced intermolecular forces between the polymer chains of the matrix. Both of these factors result in shrinking of pore sizes of the cryogel matrix.
Experimental
Materials
Polyvinyl alcohol (PVA) (98.6% hydrolyzed, Mol. Wt. 1x 105 Da) and chitosan were purchased from Merck and used without purification. The antibacterial liquid savlon was a combination of chlorohexadiene gluconate and cetrimede and purchased from Johnson Johnson. The rest of the chemicals were of analytical grade, and doubly distilled water was used throughout the experiments.
Fabrication of savlon-loaded cryogels
In order to design a polymer blend of PVA and chitosan containing savlon as an antibacterial liquid, a cyclic freeze-thaw method was followed. In a typical experimental protocol, 3g of PVA was dissolved in a calculated amount of hot water, and 0.75 g of chitosan was dissolved in 2% acetic acid separately. Then, the above two polymer solutions were mixed, and 5 mL of savlon was added to the polymer solution. The mixture was homogenized properly and kept in a Petri dish at -20°C for 24 h. The frozen gel was thawed for 2 h at room temperature and again placed for freezing. In this way, these freezing-thawing cycles were repeated a number of times so that the whole gel ultimately changed into a semi-transparent solid but soft mass. The prepared savlon-loaded cryogels were allowed to swell in water until equilibrium swelling, so that excess polymers and savlon were leached out. The cryogels were cut into circular discs of definite size and stored in air-tight polyethylene bags for further studies.
Swelling study in PBS
The quantity of water imbibed by a material is an important property, as it greatly contributes to biocompatibility of the end material and decides if the material may be used for biomedical purposes. In order to access the water sorption potential of prepared cryogels, the dried gels were immersed in phosphate buffered saline (PBS) for a defined time period, taken out and gently pressed in between the filter papers and weighed. The water sorption was quantified in terms of swelling ratio as given below (eq. 1),
Swelling ratio =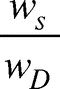
where Ws and WD are the swollen and dry weights of the gels, respectively.
Deswelling study
The capacity of the cryogels to retain imbibed water was judged by conducting a deswelling study of the prepared gels. For this purpose, at first, the gels were swollen in PBS until equilibrium and thereafter allowed to loose water by placing them under open atmosphere. The amount of water lost by the cryogels was monitored at desired and predetermined time intervals after wiping off their surfaces using filter papers. The percent deswelling was calculated by equation 2,
% deswelling = 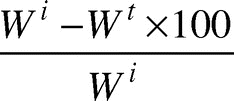
where Wi and Wt are the weights of swollen gels at time zero time (start) and time t, respectively.
Charactarization
FTIR spectral analysis
The structural confirmation of the fabricated cryogels determined by recording their FTIR spectra on an IR spectrophotometer (Perkin Elmer, 1000 Paragon).
ESEM analysis
Environmental scanning electron microscopy (ESEM) is a powerful technique to gain insights into the morphology of the materials’ surfaces. This method of recording surface images of a material differs from the classical SEM machine in the respect that no sample preparation is needed in this technique in comparison to the latter method, where sample preparation is essential. In the present study, the samples of the cryogels were dried in vacuum desiccators and put on the ESEM machine (Quanta 200, Icon).
In vitro blood compatibility
The in vitro evaluation of blood compatibility of a material provides a preliminary idea of the material, whether that can be employed for biological purposes or not. In the present investigation, therefore, some methods have been followed to judge the in vitro blood compatibility of the prepared cryogels, as discussed below.
Clot formation test
Blood clot formation on the surface of a material presents a direct consequence of the interaction between the material and blood components. In the present study, the antithrombogenic potential of the cryogel surfaces was judged by the blood clot formation test, as described elsewere.Citation35 In a typical experiment, the cryogel samples of varying compositions were equilibrated in saline water (0.9% w/v NaCl) at 37°C for 24 h. and 0.5 mL of ACD blood and 0.03 mL of CaCl2 solution (4 mol L−1) were added to these swollen specimens to start the thrombus formation. 4.0 mL of deionized water was added to stop the reaction, and the thrombus formed was separated by soaking the specimen in water for 10 min at room temperature then fixing in 36% formaldehyde solution (2.0 mL) for another 10 min. The fixed clot was placed in water for 10 min, and after drying, its weight was recorded. The same procedure was repeated for a glass surface, blood bags and for gels of varying compositions, and respective weights of thrombus formed were recorded.
Percent hemolysis tests
Hemolysis is another technique that gives an idea about the antithrombogenic nature of the material and provides preliminary results about the possible use of the polymers to be employed as biomaterials. In order to perform hemolysis tests, a method published elsewhere was adopted.Citation36 In a typical experiment, 0.25 mL of human ACD blood was added to dry cryogels pieces (4 cm2), which were already equilibrated with saline water (0.9% w/v NaCl) for 24 h at 37°C. The hemolysis was allowed to take place for 20 min, and thereafter, 2.0 mL of saline water was added to the specimens to stop hemolysis. The samples were incubated for 60 min at 37°C. Positive and negative controls were obtained by adding 0.25 mL of human ACD blood and 0.9% NaCl solution, respectively, to 2.0 mL of bidistilled water. Incubated samples were centrifuged for 45 min; the supernatant was taken, and its absorbance was recorded at 545 nm. The percentage hemolysis was calculated using the following relationship (eq. 3),
Hemolysis (%) =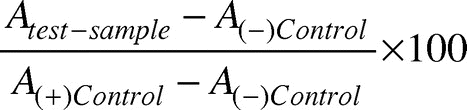
where A is absorbance. The absorbance of positive and negative controls was found to be 1.915 and 0.014, respectively.
Antibacterial assay
The study was performed using zone of inhibition method,Citation37 as described by WHO 2003. Nutrient agar plates (5 g peptone, 5 g NaCl, 3 g Beef extract, 1000 ml, Agar 2%) were prepared and seeded with 200 µL of test bacteria. Discs of cryogel of different concentration were prepared with the help of sterile cork borer (4 mm) and transferred on to the inoculated plates. They were incubated at 37°C for 24 h. The zone of inhibition was determined by measuring the diameter in millimeters of zone to which the disinfectant inhibited the growth of the organism. Three replicates of each test were conducted.
Statistical analysis
All experiments were done at least three times, and figures and data have been expressed along with the respective error bars and standard deviations.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Boateng JS, Matthews KH, Stevens HN, Eccleston GM. Wound healing dressings and drug delivery systems: a review. J Pharm Sci 2008; 97:2892 - 923; http://dx.doi.org/10.1002/jps.21210; PMID: 17963217
- Kokabi M, Sirousazar M, Hassan ZM. PVA–clay nanocomposite hydro-gels for wound dressing. Eur Polym J 2007; 43:773 - 81; http://dx.doi.org/10.1016/j.eurpolymj.2006.11.030
- Cheng CL, Koo MWL. Effects of Centella asiatica on ethanol induced gastric mucosal lesions in rats. Life Sci 2000; 67:2647 - 53; http://dx.doi.org/10.1016/S0024-3205(00)00848-1; PMID: 11104366
- Purna SK, Babu M. Collagen based dressings--a review. Burns 2000; 26:54 - 62; http://dx.doi.org/10.1016/S0305-4179(99)00103-5; PMID: 10630321
- Singh B, Pal L. Development of sterculia gum-based wound dressings for use in drug delivery. Eur Polym J 2008; 44:3222 - 30; http://dx.doi.org/10.1016/j.eurpolymj.2008.07.013
- Hamidi M, Azadi A, Rafiei P. Hydrogel nanoparticles in drug delivery. Adv Drug Deliv Rev 2008; 60:1638 - 49; http://dx.doi.org/10.1016/j.addr.2008.08.002; PMID: 18840488
- Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev 2002; 54:3 - 12; http://dx.doi.org/10.1016/S0169-409X(01)00239-3; PMID: 11755703
- Liu J, Lin S, Li L, Liu E. Release of theophylline from polymer blend hydrogels. Int J Pharm 2005; 298:117 - 25; http://dx.doi.org/10.1016/j.ijpharm.2005.04.006; PMID: 15908149
- Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm 2000; 50:27 - 46; http://dx.doi.org/10.1016/S0939-6411(00)00090-4; PMID: 10840191
- Sokolsky-Papkov M, Agashi K, Olaye A, Shakesheff K, Domb AJ. Polymer carriers for drug delivery in tissue engineering. Adv Drug Deliv Rev 2007; 59:187 - 206; http://dx.doi.org/10.1016/j.addr.2007.04.001; PMID: 17540473
- Nuttelman CR, Mortisen DT, Henery SM, Anseth KS. Attachment of fibronection to poly (vinyl alcohol) hydrogels promotes NIH3T3 cell adhesion, proliferation, and migration. J Biomed Mater Res 2001; 52:217 - 23; http://dx.doi.org/10.1002/1097-4636(200111)57:2<217::AID-JBM1161>3.0.CO;2-I
- Hassan CM, Peppas NA. Structure and application of Poly (vinyl alcohol) hydrogels produced by conventional cross-linking or by freezing-thawing methods. Adv Polym Sci 2000; 153:37 - 66; http://dx.doi.org/10.1007/3-540-46414-X_2
- Mansur HS. Costa Jr, Barbosa-stancioli EF. Cytocompatibility evaluation in cell culture systems of chemically cross-linked chitosan/pva hydrogels. Mater Sci Eng C 2009; 29:1574 - 83; http://dx.doi.org/10.1016/j.msec.2008.12.012
- Liu Y, Geever LM, Kennedy JE, McGuinness GB. Thermal behaviour and mechanical properties of physically cross-linked PVA/Geletin hydrogels. J Mech Materials 2010; 3:203 - 9
- Mc Gann MJ, Higginbotham CL, Geever LM, Nugent MJD. The synthesis of novel pH-sensitive poly(vinyl alcohol) composite hydrogels using a freeze/thaw process for biomedical applications. Int J Pharm 2009; 372:154 - 61; http://dx.doi.org/10.1016/j.ijpharm.2009.01.008; PMID: 19429275
- Nugent MJD, Higginbotham CL. Preparation of a novel freeze thawed poly(vinyl alcohol) composite hydrogel for drug delivery applications. Eur J Pharm Biopharm 2007; 67:377 - 86; http://dx.doi.org/10.1016/j.ejpb.2007.02.014; PMID: 17398082
- Lim SH, Hudson SM. Review of chitosan and its derivatives as antimicrobial agents and their uses as textile chemical. Pol Rev 2003; 43:223 - 69
- Burkatovskaya M, Tegos GP, Swietlik E, Demidova TN, P Castano A, Hamblin MR. Use of chitosan bandage to prevent fatal infections developing from highly contaminated wounds in mice. Biomaterials 2006; 27:4157 - 64; http://dx.doi.org/10.1016/j.biomaterials.2006.03.028; PMID: 16616364
- Tang H, Zhang P, Kieft TL, Ryan SJ, Baker SM, Wiesmann WP, et al. Antibacterial action of a novel functionalized chitosan-arginine against Gram-negative bacteria. Acta Biomater 2010; 6:2562 - 71; http://dx.doi.org/10.1016/j.actbio.2010.01.002; PMID: 20060936
- Wittaya-areekul S, Prahsarn C. Development and in vitro evaluation of chitosan-polysaccharides composite wound dressings. Int J Pharm 2006; 313:123 - 8; http://dx.doi.org/10.1016/j.ijpharm.2006.01.027; PMID: 16488564
- Wu YB, Yu SH, Mi FL, Wu CW, Shyu AC, Peng CK, Chao AC, Preparation and Characterization on mechanical and antibacterial properties of chitosan/cellulose blend, Carbohydr. Polymers 2004; 57: AC 435-440.
- Huang H, Gu L, Ozaki Y. Non-isothermal crystallization and thermal transitions of a biodegradable, partially hydrolyzed poly (vinyl alcohol). Polymer (Guildf) 2006; 47:3935 - 45; http://dx.doi.org/10.1016/j.polymer.2006.03.089
- Hernandez R, Sarafian A, Lopez D, Mijangos C. Viscoelastic properties of poly(vinyl alcohol) hydrogels and ferrogels obtained through freezing-thawing cycles. Carbohydr Polym 2004; 46:5543 - 9
- Huang MH, Yang MC. Evaluation of glucan/poly(vinyl alcohol) blend wound dressing using rat models. Int J Pharm 2008; 346:38 - 46; http://dx.doi.org/10.1016/j.ijpharm.2007.06.021; PMID: 17662545
- Mc Gann MJ, Higginbotham CL, Geever LM, Nugent MJD. The synthesis of novel pH-sensitive poly(vinyl alcohol) composite hydrogels using a freeze/thaw process for biomedical applications. Int J Pharm 2009; 372:154 - 61; http://dx.doi.org/10.1016/j.ijpharm.2009.01.008; PMID: 19429275
- Kenawy ER, Mohamed H. El-Newehy, Salem S. Al-Deyab, Controlled release of atenolol from freeze/thawed poly(vinyl alcohol) hydrogel. J Saudi Chem Soc 2010; 14:237 - 40; http://dx.doi.org/10.1016/j.jscs.2010.02.014
- Bolto B, Tran T, Hoang M. Xie Zi, Cross-linked poly(vinylalcohol) mambrane. Prog Polym Sci 2009; 34:969 - 81; http://dx.doi.org/10.1016/j.progpolymsci.2009.05.003
- Smith TJ, Kennedy JE, Higginbotham CL. The rheological and thermal characteristics of freeze-thawed hydrogels containing hydrogen peroxide for potential wound healing applications. J Mech Behav Biomed Mater 2009; 2:264 - 71; http://dx.doi.org/10.1016/j.jmbbm.2008.10.003; PMID: 19627831
- Bajpai AK, Saini R. Preparation and characterization of novel biocompatible cryogels of poly (vinyl alcohol) and egg-albumin and their water sorption study. J Mater Sci Mater Med 2006; 17:49 - 61; http://dx.doi.org/10.1007/s10856-006-6329-z; PMID: 16389472
- Singh A, Narvi SS, Dutta PK, Pandey ND. External stimuli response on a novel chitosan hydrogel cross-linked with formaldehyde. Bull Mater Sci 2006; 29:233 - 8; http://dx.doi.org/10.1007/BF02706490
- Young AM, Poon Yun J. Ng, Gbureck U, Nazhat SN, Barralet JE, Hofman MP. Charaeterization of chlorohexidine-releasing, fast-setting, brushite bone cements. Acta Biomater 2008; 4:1081 - 8; http://dx.doi.org/10.1016/j.actbio.2007.12.009; PMID: 18313374
- Bagri LP, Bajpai J, Bajpai AK. Cryogenic Designing of Biocompatible Blends of Polyvinyl alcohol and Starch with Macroporous Architecture. J Macromol Sci Pure Appl Chem 2009; 46:1060 - 8; http://dx.doi.org/10.1080/10601320903252025
- Bajpai AK, Mishra A. Preparation and Characterization of tetracycline-loaded Interpenetrating polymer networks of carboxymethyl cellulose and poly (acrylic Acid): water sorption and drug delivery study. Polym Int 2005; 54:1347 - 56; http://dx.doi.org/10.1002/pi.1839
- Bajpai AK, Saini R. Preparation and characterization of biocompatible spongy cryogels of poly (vinyl alcohol)-gelatin and study of water sorption behaviour. Polym Int 2005; 54:1233 - 42; http://dx.doi.org/10.1002/pi.1813
- Maitz MF, Pham MT, Wieser E, Tsyganov I. Blood compatibility of titanium oxides with various crystal structure and element doping. J Biomater Appl 2003; 17:303 - 19; http://dx.doi.org/10.1177/0885328203017004005; PMID: 12797422
- Bajpai AK, Kankane S. Preparation and characterization of Macroporous Poly (2-hydroxyethyl methacrylate) based biomaterials; water sorption property and invitro blood compatibility. J Appl Polym Sci 2007; 104:1559 - 71; http://dx.doi.org/10.1002/app.25580
- Awodele E, Agbamuche PM, Akintonwa A. The antimicrobial activ481 ities of some commonly used disinfectants on Bacillus subtilis, Pseudomonas 482 aeruginosa and Candida albicans. Afr J Biotechnol 2007; 6:987 - 90