Abstract
It has long been known that the interfacial relationship between synthetic materials and tissue is influential in the success of implant materials. Instability at the implant interface has been shown, in some cases, to lead to complete implant failure. Bioceramics, and in particular calcium phosphates, form a large fraction of the implantable devices on the market today due to the biocompatibility they exhibit in contact with bone and tooth-like tissues. The characterization of such bioceramic-tissue interfaces has played a crucial role in understanding the behavior of bioceramics in vivo. In this review, we shed light on the preparation methods, technological approaches and key advances in resolving the interface between calcium phosphate bioceramics and bone, and share a future outlook on this field.
Introduction
One of the most thoroughly investigated categories of bioceramics is calcium phosphates. This broad term envelops materials such as hydroxyapatite (HA), tricalcium phosphates, tetracalcium phosphates, octacalcium phosphates (OCP) and ion-substituted forms of the aforementioned. Calcium phosphate ceramics have vast applications in the biomedical field due to their similar composition to the mineral component of bone. Bone mineral is believed to be a combination of HA and ion-substituted apatites.Citation1-Citation4 The calcium phosphate interface in vivo is dynamic, particularly at the biological side, thereby allowing bone formation both into and from the CaP surface.Citation5,Citation6
Calcium phosphates for bone regrowth and regeneration are available in a number of forms including granules, blocks, injectable cements, scaffolds and coatings.Citation3,Citation7 Of the properties associated with these bioceramics, biocompatibility is perhaps one of the most important factors to consider. Biocompatibility was traditionally defined as the ability of a material to elicit an appropriate host response.Citation8,Citation9 In the case of implants intended for contact with hard tissue, such as bone, the appropriate host response involves osseointegration; the direct contact between living bone and implant surface.Citation10 Originally, when this term was defined, the requirement for contact was on the light microscopic resolution level. However, electron microscopy enables evaluation of materials at a much higher resolution level and allows determination of direct contact on a nanometer or ultrastructural level. As a result, we have seen an evolution in the understanding of the bioceramic-bone interface in accordance with the progression of characterization techniques, from light optical microscopy (LM), to scanning electron microscopy (SEM), to transmission electron microscopy (TEM) and focused ion beam microscopy (FIB). Herein, we focus mainly on the role of light and electron microscopy in the comprehension of the calcium phosphate bioceramic interface to bone in vivo.
Sample Preparation Approaches
The greatest challenge in resolving the interface between biomaterials and bone remains sample preparation. Methods to prepare samples vary widely depending on the requirements of the characterization technique. Indeed, as we move from LM to SEM to TEM the complexity of the technique, and therefore the associated sample preparation method, increases.
In all cases, the first challenge is removing an intact sample from the in vivo environment. Depending on implant geometry and location, standard removal approaches include trephine drill or low speed saw. Implementing precautionary measures, to reduce heat and mechanical forces, by the use of low-speed instruments and flushing with cool aqueous solutions can aid in the removal of intact tissue-implant specimens. As mentioned, the subsequent sample preparation procedures vary depending on the characterization technique that follows.
Tissue processing
Most samples investigated by light and SEM require embedding in resin or paraffin wax. The standard biological tissue processing method involves the sequential fixation in glutaraldehyde or formaldehyde, post-fixation in osmium tetroxide, dehydration in ethanol or acetone, followed by embedding in epoxy or acrylic based resins including PMMA or paraffin.Citation11-Citation13 The choice of embedding medium may be an important factor to consider since it may influence the degree of tissue-implant interfacial separation. For instance, the use of Technovit and Epon as embedding media have shown significantly less separation than LR White.Citation14 Similarly, the choice of embedding media also determines the thickness of sections that can be prepared for subsequent LM or EM investigations. Due to the softness of paraffin compared with plastic resins, sections of comparable thinness are not achievable.Citation15 Additionally, paraffin embedding often requires decalcification and recent investigations have shown that calcified bone embedded in Technovit provides superior preservation of trabecular bone structure and yields stronger immunostaining.Citation16
Sectioning, grinding and staining
In their article on the Säge-Schliff (sawing and grinding) technique, Donath and Breuner have outlined a method for the preparation and sectioning of implant materials in contact with bone for histological and morphological evaluation by LM.Citation17 Using this procedure or variations of it, the resultant embedded bone-implant specimens are 5–10 μm thick with the possibility of applying stains or immunogold labeling.Citation18,Citation19 In LM and SEM, staining of bone samples, with for example toluidine blue, Alizarin red, lead citrate or other heavy metals, is common practice for evaluating the stages of bone growth or improving contrast.Citation20-Citation22 Furthermore, immunogold labeling plays a key role in detecting the presence of bone specific matrix proteins such as fibronectin and osteonectin at an implant interface. Preparation of sections in this manner has enabled LM and SEM of the interface between calcified tissues and implant, allowing histological and morphometric evaluation of the short- and long-term tissue response to implanted materials.
Ultramicrotomy
To proceed with investigations of the tissue-material interface on the nanometer range, it is essential that samples are electron transparent for TEM studies. In the case of biomaterials, electron transparency corresponds to a minimum thickness between 50–100 nm.Citation23 Achieving such specimens has traditionally been done by ultramicrotomy, the slicing of thin samples with a diamond blade followed by their collection onto a TEM grid. Maintaining the contact between soft biological tissues and brittle ceramics, while maintaining the integrity of biological shapes, is often a challenge with this technique. As a result, ion milling techniques have become the preferred choice for TEM sample preparation of calcium phosphates interfacing to bone.
Ion milling
One alternative technique to ultramicrotomy for producing ultrathin electron transparent specimens is broad beam ion milling. The comparison between broad beam ion milling and FIB milling, discussed below, has shown little difference in the quality of resultant specimens.Citation24 Similarly to ultramicrotomy, the drawback of broad beam ion milling is the inability to select the exact interface region of interest, and therefore there is an associated risk that not all specimens produced will contain the region of interest within them.
Focused ion beam (FIB)
FIB instruments have been used widely in the materials science and microelectronics industry.Citation25 However, the use of dual beam FIB and SEM is gaining popularity in the life sciences. For the preparation of biomaterial-bone interfaces, the FIB presents several advantages to conventional preparation using ultramicrotomy or broad beam ion techniques. First, the coupling of the ion beam with a SEM instrument enables the site-specific selection of interfaces for study by TEM. In addition, the ion beam current or energy can be reduced sufficiently to maintain intact interfaces throughout preparation. This is particularly a challenge in ultramicrotomy, where materials of considerably different hardness values often separate under the cutting blade.
FIB sample preparation has been successfully demonstrated for surface analysis of implant materials,Citation26,Citation27 the interfaces between titanium and bone,Citation28,Citation29 titanium with magnetron sputtered HA and bone,Citation30 cells and HA,Citation31 HA and bone,Citation32 glass ionomer cements and dentin,Citation24 calcium aluminate coatings and boneCitation14 and pulsed-laser deposited HA on titanium,Citation33 to name a few. In all these examples, the FIB instrument produced samples of quality equivalent or greater than that of broad beam, tripod polished or ultramicrotomed samples, and did so while maintaining contact between materials of varying hardness.
Nevertheless, it is important to note that there are a number of artifacts associated with the use of FIB instruments.Citation34-Citation36 Although precautions should be taken to avoid ion beam-induced damage, it is possible that a small amount of gallium ion implantation may occur, which is problematic if quantitative chemical analysis by TEM methods is of concern. Moreover, differential sputtering rates and ion channeling may result in a ‘theater curtain’ effect across the sample, consisting of vertical striped regions corresponding to different milling rates, which may distort features of interest.Citation34 Lastly, redeposition of sputtered material can occur on the sample surface if the beam dose is too high, not accurately placed or if the beam is not finely tuned.Citation34 Experience, a carefully tuned instrument and, more recently, the use of low energy ion cleaning steps may help reduce the likelihood of artifact occurrences.Citation37,Citation38
Although not used for the study of bioceramics, Giannuzzi et al. have demonstrated the ability to use FIB sequential milling and imaging to produce three dimensional reconstructions of biomaterial-bone interfaces.Citation39 Such three dimensional interfacial information was useful for determining the extent of bone ingrowth into implanted titanium samples, and could easily be applied to the bioceramic interface in much the same way.
Cryo-preparation
The use of cryogenic-based techniques is quite common in the study of biological tissues. Cryofracturing has been used by Steflik et al. to ensure interfacial tissues remain intact on tissue blocks when removing implants from apposing tissue.Citation40 Of late, advancements in cryo-FIBSEM and cryo-TEM instrumentation have enabled the complete preparation, transfer and investigation of biological specimens from start to finish under cryogenic conditions, eliminating the need for rigorous tissue processing.Citation41 This may in fact be the best route for preparing and examining biological structures in their native state.
Resolving the Interface: The Techniques
Light microscopy (LM)
Ground sections must be produced, as described earlier, for light microscopic evaluation of the intact interface between non-demineralized tissue and implants, so that features such as bone-implant contact and bone area can be quantified. It has been shown that the thickness of the ground sections are of importance for quantification, as thicker samples include more overlapping information and often result in an over-estimation of bone implant contact.Citation42 Furthermore, the cutting direction may influence the quality of sections, thereby also interfering with quantification.Citation43 Different staining protocols enable the identification of features for qualitative histology, such as discrimination of woven bone tissue, mature bone tissue, as well as cellular activity. Additionally, with the injection of calcium binding dyes during healing, the mineralization front can be tracked in the ground-section, contributing information regarding, e.g., the origin of bone tissue growth. Linear or circular polarized light microscopy, covered comprehensively elsewhere,Citation44 may also be employed to identify the orientation of collagen in bone, enabling identification of regions of woven or lamellar bone in contact with calcium phosphates.Citation45
Scanning electron microscopy (SEM)
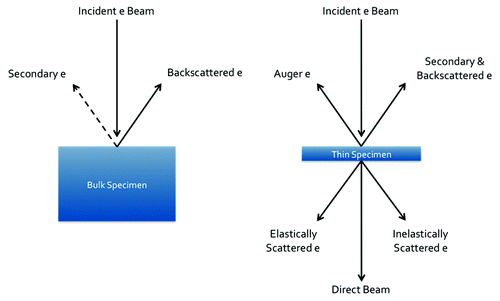
The scanning electron microscope is a valuable characterization tool for bone-implant interfacial analysis. Of the variety of electron-matter interactions that occur in the SEM (), the detection of backscattered electrons is the most useful in the study of calcium phosphates and bone. Backscattered electrons are highly Z-dependent and therefore create Z-contrast images. This is crucial for studying calcium phosphates in contact with bone, since their chemical similarities make them difficult to distinguish otherwise. The gold standard in determining percentage of bone-implant contact and bone-in-growth area is backscattered SEM.Citation46 In addition to its application for bone contact measurements, backscattered electrons are useful for choosing sites of good bone-implant contact for further investigation with TEM. SEM can also be used to obtain analytical information from the sample by collecting the characteristic X-rays emitted with energy dispersive X-ray spectroscopy.
Transmission electron microscopy (TEM)
In the TEM, electrons are transmitted through an extremely thin (≤ 100 nm) sample. A variety of signals can be detected () and therefore the TEM has a number of different techniques that are useful for the analysis of bioceramic-bone interfaces. Imaging can be achieved using bright or dark-field TEM (BFTEM, DFTEM) or high-angle annular dark-field (HAADF) in scanning transmission electron microscopy (STEM). Since the HAADF detector collects incoherently scattered electrons, the image is highly Z-dependent and thus images with compositional contrast are attainable.
Elemental analysis of samples can be achieved via many routes in the TEM including energy dispersive X-ray spectroscopy (EDS), energy filtered TEM (EFTEM) or electron energy loss spectroscopy (EELS). Gregori et al. demonstrated that EELS is an effective method to differentiate between individual grains of HA and β-tricalcium phosphate by analyzing the oxygen K ionization edge.Citation47
Atomic force microscopy (AFM)
Scanning probe microscopies rely on the interaction between a physical probe and the sample surface and indeed have been applied to the study of various biomaterial interfaces. AFM is capable of producing high-resolution images, comparable to that of TEM, without the requirement of electron transparency. Since the technique is extremely surface sensitive, sample preparation of interfaces remains a challenge. For example, Yoshida et al. demonstrated the success of diamond knife cutting vs. polishing in their study of the dentin-resin interface with AFM.Citation48 Other studies have shown the potential of AFM as a tool to study biomechanical properties, such as Young’s Modulus, at the interface between titanium and bone.Citation49 AFM has played an influential role in understanding mechanisms at the bioactive glass surface in vitro, for example, tracking the growth, distribution and roughness of globular like deposits formed in serum-free and serum-containing solutions.Citation50 AFM remains a relatively unexplored instrument in the study of bioceramic-bone interfaces. However, permitting the proper surface conditions, AFM offers a high-resolution alternative to TEM.
Resolving the HA-Bone Interface: Discoveries with LM and EM
LM fluorochrome labeling has indicated that bone formation away from the implant surface is 30% faster than formation of bone toward the implant, which signifies the importance of understanding the HA surface in vivo.Citation51 A variety of competing theories regarding the mechanisms of bone growth, the effect of properties such as crystallinity and porosity and the presence of an interfacial apatite layer, exist. These are discussed further.
Mechanisms of bone growth at the HA surface
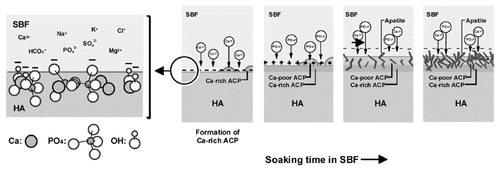
It is well known that HA is a bioactive material, in that it precipitates an apatite layer on its surface in vivo, enabling it to form a chemical bond with bone.Citation2 Such bone bonding capabilities are of particular interest in the bone regeneration field. The proposed mechanism for bone bonding is a dissolution-reprecipitation process to form a biologically active apatite layer on the HA surface.Citation5,Citation52-Citation54 Ducheyne et al. have outlined the possible processes occurring on the surface of an HA sample in vivo. Eleven interactions occur simultaneously on the implant surface: (1) dissolution from the ceramic; (2) precipitation from solution onto the ceramic; (3) ion exchange and structural rearrangement at the ceramic-tissue interface; (4) interdiffusion from the surface boundary layer into the ceramic; (5) solution-mediated effects on cellular activity; (6) deposition of either (a) the mineral phase or (b) the organic phase, without integration into the ceramic surface; (7) deposition with integration into the ceramic; (8) chemotaxis to the ceramic surface; (9) cell attachment and proliferation; (10) cell differentiation; and (11) extracellular matrix formation.Citation52 Since the in vivo situation, as demonstrated, is extremely complex, many simplifications have been drawn by studying HA in vitro. In simulated body fluid solution, the formation of surface apatite is easily explained by the recruitment of calcium and phosphorus ions from the medium to the implant material due to the surface charge, forming an amorphous calcium phosphate layer illustrated in .Citation55 Furthermore, recent advanced cryo-TEM and cryo-electron tomography techniques have uncovered the sequence of biomineralization in vitro, suggesting that the formation of pre-nucleation clusters proceeds the formation of amorphous apatite.Citation56 From these studies, we gain insight into processes that could occur in an in vivo environment.
Figure 2. Schematic diagram representing the formation of amorphous calcium phosphate apatite on the surface of HA in vitro. Reproduced with permission from reference Citation55.
Effect of materials properties
If we accept that initial bone growth is governed by a dissolution-reprecipitation method, then it is important to understand the dissolution behavior of calcium phosphates in vivo. A number of factors influence the rate of dissolution such as composition, crystallinity, grain size/boundaries and porosity.
Crystallinity
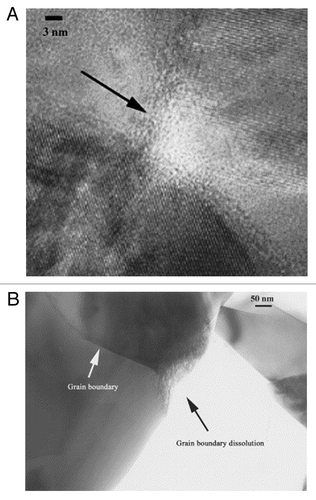
Upon dissolution in vivo, the interface takes on a jagged, rough surface exhibiting increased dissolution at grain boundaries ().Citation57,Citation58 In addition, reduced crystallinity has been noted in the vicinity of triple point grain boundary junctions ().Citation58 Annealed and crystalline coatings of plasma-sprayed HA have been shown to take longer to produce crystallites on their surface vs. a less crystalline coating, likely due to the reduced solubility of more crystalline materials.Citation59 Daculsi et al. also showed that line defects, representing a disruption in crystallinity, acted as starting points for dissolution of both biological and synthetic apatites.Citation54
Figure 3. (A) EFTEM micrograph showing the reduced crystallinity at a triple-point junction in Si-substituted HA, (B) TEM micrograph exhibiting increased dissolution at a grain boundary. Reproduced with permission from reference Citation58.
Porosity
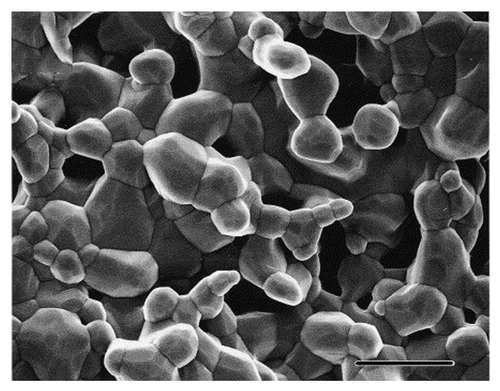
It is well accepted that a macroporous structure of 100–400 μm is required to promote bone ingrowth.Citation53 In addition, Jones et al. have identified a cut-off accessible pore radius of 100 μm for cellular activity required to promote bone growth.Citation60,Citation61 However, the presence of micropores is of increasing importance in the development of bone ingrowth. With SEM, micropores of 5 μm are regularly noted in HA samples, and generally located at grain boundaries (). It has been shown that the presence of these micropores may aid in resorption or dissolution by macroscopic break up rather than phagocytosis.Citation53 With the aid of LM, it has been demonstrated that, in otherwise identical macroporous scaffolds, the presence of microporosity increases bone growth.Citation62
Figure 4. SEM micrograph of HA showing micropores and grain boundaries that contribute to increased dissolution and biocompatibility. Scale bar is 5 μm. Reproduced with permission from reference Citation71.
Chemistry
The doping of HA with Si has been found to destabilize the HA crystal structure, and reduce grain size, thereby increasing dissolution rates which result in increased bone apposition.Citation41,Citation58 Interestingly, other studies have indicated that the behavior of various tricalcium phosphates, tetracalcium phosphates and HA samples in vivo is irrespective of their calcium to phosphorous ratio.Citation63 Furthermore, it has been noted that the ceramic behavior in vivo is irrespective of implant site, species and implantation time.Citation64
The interfacial layer
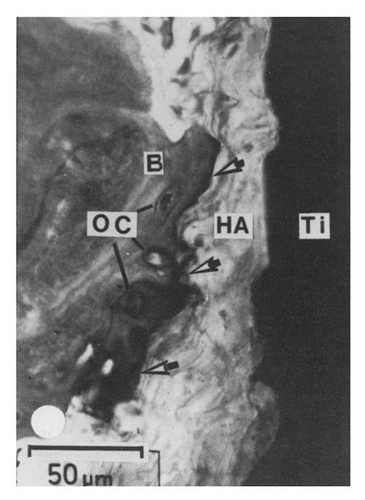
To date, a consensus on the exact behavior of calcium phosphates in vivo, and in particular HA, has not been reached in the scientific community. Competing views on the surface formation of an interfacial apatite layer, its composition and its orientation with respect to bioceramic implant, exist. The surface behavior of HA in various forms has been studied thoroughly. Light microscopic techniques have shown evidence of a dark non-collagenous interfacial zone ().Citation45 Similarly, many TEM studies have identified a collagen free layer,Citation65 ranging in thickness from 20–1000 nm.Citation52,Citation65,Citation66 The composition of this layer has been suggested to be the inorganic matrix of bone and non-collagenous proteins. Steflik et al. identified interdigitating canaliculi connecting the interfacial layer to osteocytes outside the layer, indicating the presence of cellular communication between the implant interface and bone, clearly conflicting with the viewpoint that only a dissolution-reprecipitation mechanism results in the formation of the surface apatite layer.Citation40,Citation52
Figure 5. Light micrograph of the HA coated titanium to bone interface. Arrows indicate the dark interfacial region of organic, non-collagenous material. Reproduced with permission from reference Citation45.
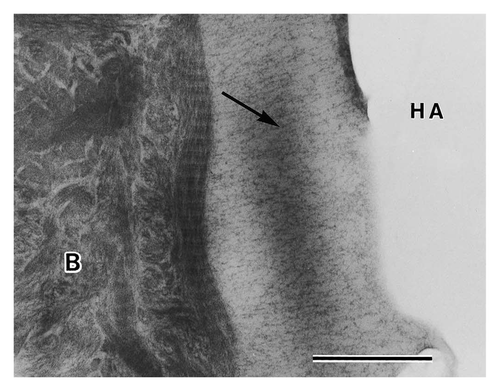
Electron diffraction has confirmed the type of precipitate that forms in this interfacial layer to be wholly, or a combination of, HA and OCP.Citation67-Citation70 Xin et al. have identified a crystallographic relationship common to OCP/HA interfaces suggesting the possibility that an in vivo shift from OCP to HA occurs.Citation70 In the instances of only HA precipitation, Hemmerle et al. have shown that in vivo HA crystal growth follows the same (110) and (001) orientation of the implanted HA, thereby suggesting that epitaxial growth occurs in vivo.Citation67 Daculsi et al. have also shown crystal growth perpendicular to the surface of the ceramic and electron diffraction confirmed that the c-axes of newly formed precipitates were in agreement with those of the originally implanted material.Citation64 Furthermore, Fujita et al. showed a fibrillar structure formed perpendicular to the implant surface ().Citation71
Figure 6. TEM micrograph of an HA particle after 4 weeks in rats. Precipitates are forming perpendicular to the crystal surface indicated by an arrow. Scale bar is 0.5 μm. Reproduced with permission from reference Citation71.
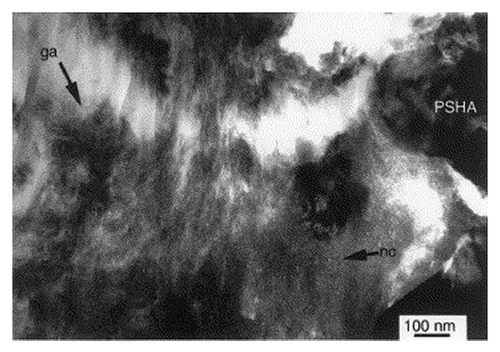
New developments in TEM techniques have enabled three-dimensional imaging of nanostructures. Through the use of electron tomography, the compilation and reconstruction of many images taken over a large angular range, the HA-bone interface has also shown perpendicular crystals in the interfacial zone (), while EDS shows a compositional gradient suggesting their formation by a dissolution-reprecipitation mechanism.Citation32 However, other investigations suggest that crystal formation is not always perpendicular. In the case of a plasma-sprayed HA coating, Porter et al. identified a nanocrystalline region and the alignment of HA crystals parallel to the interface after 10-day implantation in dogs ().Citation59
Figure 7. Electron tomograms indicating the orientation of HA crystallites in bone parallel to the HA surface, while crystallites precipitated at the scaffold interface are perpendicular to the surface. Reproduced with permission from reference Citation72.
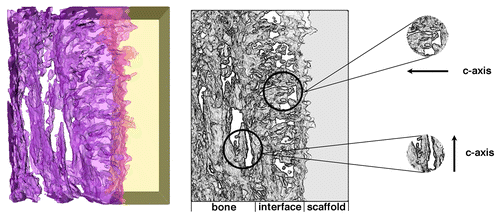
Figure 8. TEM micrograph of bone interfacing to plasma-sprayed HA. A nanocrystalline region is shown (labeled nc) and alignment of HA crystals appears parallel to the PSHA interface. Reproduced with permission from reference Citation59.
Light and electron microscopic investigations have provided strong evidence to support the behavior of HA in vivo. While the exact composition and orientation of precipitate formation remains disputed, it is generally accepted that an apatite layer forms on the surface of HA in vivo to facilitate bone growth and attachment.
Conclusions and Future Outlook
Resolving the interface between calcium phosphate materials and bone has been possible through the advancements in microscopy. Techniques such as light, scanning electron, focused ion beam, scanning probe and transmission electron microscopy have all played a role in resolving the bioceramic-bone interface. Indeed, the evolution from light to electron microscopic techniques has enabled a greater understanding of the calcium phosphate to bone interface by providing structural and elemental information on the nanometer or ultrastructural level. While a considerable number of advances have been made in the last decade to improve the ability to resolve the interface between bioceramics and bone, a number of improvements remain to be made. Recent developments enabling three-dimensional interfacial imaging are on the forefront of bioceramic-bone analysis. Our work with Z-contrast electron tomography has demonstrated the usefulness of imaging an interface at both the nanometer scale and in three dimensions, where the resulting tomograms from an HA-bone interface provided a greater amount of information than that obtained purely from two-dimensional projections.Citation72 Moreover, a paradigm shift in interfacial imaging encourages researchers to aim for non-invasive and non-destructive imaging techniques that provide increased clinical relevance. These aims have led to the increased use of X-ray computed tomography (CT).Citation60,Citation73,Citation74 In particular, the increased availability of systems such as the NanoSpect/CT has revolutionized the ability to image small-animal bone-implant interfaces in a timely manner.Citation75 With continual developments in microscopy techniques and capabilities, new discoveries and revelations of the calcium phosphate to bone interface are on the horizon.
| Abbreviations: | ||
| AFM | = | atomic force microscopy |
| BFTEM | = | bright-field transmission electron microscopy |
| Cryo-FIBSEM | = | cryogenic focused ion beam scanning electron microscopy |
| Cryo-TEM | = | cryogenic transmission electron microscopy |
| CT | = | computed tomography |
| DFTEM | = | dark-field transmission electron microscopy |
| EDS | = | energy dispersive X-ray spectroscopy |
| EELS | = | electron energy loss spectroscopy |
| EFTEM | = | energy-filtered transmission electron microscopy |
| EM | = | electron microscopy |
| FIB | = | focused ion beam |
| HA | = | hydroxyapatite |
| HAADF | = | high-angle annular dark-field |
| LM | = | light microscopy |
| OCP | = | octacalcium phosphate |
| SEM | = | scanning electron microscopy |
| STEM | = | scanning transmission electron microscopy |
| TEM | = | transmission electron microscopy |
References
- LeGeros RZ. Calcium phosphate-based osteoinductive materials. Chem Rev 2008; 108:4742 - 53; http://dx.doi.org/10.1021/cr800427g; PMID: 19006399
- LeGeros RZ. Properties of osteoconductive biomaterials: calcium phosphates. Clin Orthop Relat Res 2002; 395:81 - 98; http://dx.doi.org/10.1097/00003086-200202000-00009; PMID: 11937868
- Vallet-Regi M, Gonz´lez-Calbet JM. Calcium phosphates as substitution of bone tissues. Prog Solid State Chem 2004; 32:1 - 31; http://dx.doi.org/10.1016/j.progsolidstchem.2004.07.001
- Hench LL. Bioceramics: From Concept to Clinic. J Am Ceram Soc 1991; 74:1487 - 510; http://dx.doi.org/10.1111/j.1151-2916.1991.tb07132.x
- Plenk HJ Jr. Prosthesis-bone interface. J Biomed Mater Res 1998; 43:350 - 5; http://dx.doi.org/10.1002/(SICI)1097-4636(199824)43:4<350::AID-JBM2>3.0.CO;2-S; PMID: 9855193
- Hench LL. Biomaterials: a forecast for the future. Biomaterials 1998; 19:1419 - 23; http://dx.doi.org/10.1016/S0142-9612(98)00133-1; PMID: 9794512
- Dorozhkin von S, Epple M. Biological and Medical Significance of Calcium Phosphates. Angew Chem Int Ed 2002; 41:3130 - 46; http://dx.doi.org/10.1002/1521-3773(20020902)41:17<3130::AID-ANIE3130>3.0.CO;2-1
- Williams DF. Definitions in biomaterials. Amsterdam: Elsevier, 1987.
- Williams DF. On the mechanisms of biocompatibility. Biomaterials 2008; 29:2941 - 53; http://dx.doi.org/10.1016/j.biomaterials.2008.04.023; PMID: 18440630
- Brånemark P-I, Hansson B-O, Adell R, Breine U, Lindström J, Halle´n O, et al. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand J Plast Reconstr Surg Suppl 1977; 16:1 - 132; PMID: 356184
- Qu S-X, Lu X, Leng Y. TEM Study of Bone and Scaffold Materials. In: Qin L, Genant HK, Griffith JF, Leung KS, eds. Advanced Bioimaging Technologies in Assessment of the Quality of Bone and Scaffold Materials. Springer Berlin Heidelberg, 2007: 73–392.
- Spurr AR. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 1969; 26:31 - 43; http://dx.doi.org/10.1016/S0022-5320(69)90033-1; PMID: 4887011
- Sterchi DL, Eurell JAC. An Evaluation of Methylmethacrylate Mixtures for Hard Tissue Embedding. J Histotechnol 1995; 18:45 - 9
- Palmquist A, Jarmar T, Hermansson L, Emanuelsson L, Taylor A, Taylor M, et al. Calcium aluminate coated and uncoated free form fabricated CoCr implants: a comparative study in rabbit. J Biomed Mater Res B Appl Biomater 2009; 91:122 - 7; http://dx.doi.org/10.1002/jbm.b.31380; PMID: 19402147
- Fischer A, Jacobson K, Rose J, Zeller R. Preparation of Cells and Tissues for Fluorescence Microscopy. In: Spector D, Goldman R, editors. Basic methods in microscopy: protocols and concepts from Cells: a laboratory manual. New York: Cold Spring Harbor Laboratory Press, 2006: 111–112.
- Wittenburg G, Volkel C, Mai R, Lauer G. Immunohistochemical comparison of differentiation markers on paraffin and plastic embedded human bone samples. J Physiol Pharmacol 2009; 60:43 - 9; PMID: 20400791
- Donath K, Breuner G. A method for the study of undecalcified bones and teeth with attached soft tissues. The Säge-Schliff (sawing and grinding) technique. J Oral Pathol 1982; 11:318 - 26; http://dx.doi.org/10.1111/j.1600-0714.1982.tb00172.x; PMID: 6809919
- Cano-S´nchez J, Campo-Trapero J, Gonzalo-Lafuente JC, Moreno-Lo´pez LA, Bascones-Marti´nez A. Undecalcified bone samples: a description of the technique and its utility based on the literature. Med Oral Patol Oral Cir Bucal 2005; 10:E74 - 87; PMID: 15800470
- Wallin JA, Tkocz I, Levinsen J. A simplified procedure for preparation of undecalcified human bone sections. Stain Technol 1985; 60:331 - 6; PMID: 4089889
- Yang R, Davies CM, Archer CW, Richards RG. Immunohistochemistry of matrix markers in Technovit 9100 New-embedded undecalcified bone sections. Eur Cell Mater 2003; 6:57 - 71, discussion 71; PMID: 14722903
- Venable JH, Coggeshall R. A simplified lead citrate stain for use in electron microscopy. J Cell Biol 1965; 25:407 - 8; http://dx.doi.org/10.1083/jcb.25.2.407; PMID: 14287192
- Watson ML. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol 1958; 4:475 - 8; http://dx.doi.org/10.1083/jcb.4.4.475; PMID: 13563554
- Williams DB, Carter CB. Transmission electron microscopy: a textbook for materials science. New York: Plenum Press, 1996.
- Coutinho E, Jarmar T, Svahn F, Neves AA, Verlinden B, Van Meerbeek B, et al. Ultrastructural characterization of tooth-biomaterial interfaces prepared with broad and focused ion beams. Dent Mater 2009; 25:1325 - 37; http://dx.doi.org/10.1016/j.dental.2009.06.002; PMID: 19596422
- Steve R, Robert P. A review of focused ion beam applications in microsystem technology. J Micromech Microeng 2001; 11:287; http://dx.doi.org/10.1088/0960-1317/11/4/301
- Jarmar T, Palmquist A, Brånemark R, Hermansson L, Engqvist H, Thomsen P. Characterization of the surface properties of commercially available dental implants using scanning electron microscopy, focused ion beam, and high-resolution transmission electron microscopy. Clin Implant Dent Relat Res 2008; 10:11 - 22; http://dx.doi.org/10.1111/j.1708-8208.2007.00056.x; PMID: 18254738
- Jarmar T, Palmquist A, Brånemark R, Hermansson L, Engqvist H, Thomsen P. Technique for preparation and characterization in cross-section of oral titanium implant surfaces using focused ion beam and transmission electron microscopy. J Biomed Mater Res A 2008; 87:1003 - 9; http://dx.doi.org/10.1002/jbm.a.31856; PMID: 18257067
- Palmquist A, Jarmar T, Emanuelsson L, Brånemark R, Engqvist H, Thomsen P. Forearm bone-anchored amputation prosthesis: a case study on the osseointegration. Acta Orthop 2008; 79:78 - 85; http://dx.doi.org/10.1080/17453670710014806; PMID: 18283577
- Palmquist A, Lindberg F, Emanuelsson L, Brånemark R, Engqvist H, Thomsen P. Biomechanical, histological, and ultrastructural analyses of laser micro- and nano-structured titanium alloy implants: a study in rabbit. J Biomed Mater Res A 2010; 92:1476 - 86; PMID: 19425049
- Engqvist H, Botton GA, Couillard M, Mohammadi S, Malmström J, Emanuelsson L, et al. A novel tool for high-resolution transmission electron microscopy of intact interfaces between bone and metallic implants. J Biomed Mater Res A 2006; 78:20 - 4; http://dx.doi.org/10.1002/jbm.a.30696; PMID: 16596587
- Engqvist H, Svahn F, Jarmar T, Detsch R, Mayr H, Thomsen P, et al. A novel method for producing electron transparent films of interfaces between cells and biomaterials. J Mater Sci Mater Med 2008; 19:467 - 70; http://dx.doi.org/10.1007/s10856-006-0042-9; PMID: 17607519
- Grandfield K, Palmquist A, Ericson F, Malmström J, Emanuelsson L, Slotte C, et al. Bone Response to Free-Form Fabricated Hydroxyapatite and Zirconia Scaffolds: A Transmission Electron Microscopy Study in the Human Maxilla. Clin Implant Dent Relat Res 2010; http://dx.doi.org/10.1111/j.1708-8208.2009.00270.x; PMID: 20156226
- Iliescu M, Nelea V, Werckmann J, Mihailescu IN. Transmission electron microscopy investigation of pulsed-laser deposited hydroxylapatite thin films prepared by tripod and focused ion beam techniques. Surf Coat Tech 2004; 187:131 - 40; http://dx.doi.org/10.1016/j.surfcoat.2004.01.022
- Giannuzzi LA, Stevie FA. Introduction to Focused Ion Beams. Boston: Springer Science, 2005.
- Giannuzzi LA, Stevie FA. A review of focused ion beam milling techniques for TEM specimen preparation. Micron 1999; 30:197 - 204; http://dx.doi.org/10.1016/S0968-4328(99)00005-0
- Phaneuf M. Applications of focused ion beam microscopy to materials science specimens. Micron 1999; 30:277 - 88; http://dx.doi.org/10.1016/S0968-4328(99)00012-8
- Giannuzzi L. Reducing FIB Damage Using Low Energy Ions. Microsc Microanal 2006; 12:1260; http://dx.doi.org/10.1017/S1431927606065469
- Giannuzzi LA, Geurts R, Ringnalda J. 2 keV Ga+ FIB Milling for Reducing Amorphous Damage in Silicon. Microsc Microanal 2005; 11:828 - 9; http://dx.doi.org/10.1017/S1431927605507797
- Giannuzzi LA, Phifer D, Giannuzzi NJ, Capuano MJ. Two-dimensional and 3-dimensional analysis of bone/dental implant interfaces with the use of focused ion beam and electron microscopy. J Oral Maxillofac Surg 2007; 65:737 - 47; http://dx.doi.org/10.1016/j.joms.2006.10.025; PMID: 17368372
- Steflik DE, Corpe RS, Young TR, Sisk AL, Parr GR. The biologic tissue responses to uncoated and coated implanted biomaterials. Adv Dent Res 1999; 13:27 - 33; http://dx.doi.org/10.1177/08959374990130011101; PMID: 11276743
- Richter S, Schwedt A, Edwards HK, Coe S, Tao T, Fay MW, et al. The analysis of Si doped hydroxyapatite coatings using FIBSEM, TEM and RHEED. In: EMC 2008 14th European Microscopy Congress 1–5 September 2008, Aachen, Germany. Springer Berlin Heidelberg, 2008: 731–732.
- Johansson CB, Morberg P. Importance of ground section thickness for reliable histomorphometrical results. Biomaterials 1995; 16:91 - 5; http://dx.doi.org/10.1016/0142-9612(95)98268-J; PMID: 7734653
- Johansson CB, Morberg P. Cutting directions of bone with biomaterials in situ does influence the outcome of histomorphometrical quantifications. Biomaterials 1995; 16:1037 - 9; http://dx.doi.org/10.1016/0142-9612(95)94913-6; PMID: 8580257
- Bromage TG, Goldman HM, McFarlin SC, Warshaw J, Boyde A, Riggs CM. Circularly polarized light standards for investigations of collagen fiber orientation in bone. Anat Rec B New Anat 2003; 274:157 - 68; http://dx.doi.org/10.1002/ar.b.10031; PMID: 12964206
- de Lange GL, Donath K. Interface between bone tissue and implants of solid hydroxyapatite or hydroxyapatite-coated titanium implants. Biomaterials 1989; 10:121 - 5; http://dx.doi.org/10.1016/0142-9612(89)90044-6; PMID: 2706299
- Kokubo T. Bioceramics and Their Clinical Applications. Boca Raton: CRC Press, 2008.
- Gregori G, Kleebe H, Mayr H, Ziegler G. EELS characterisation of β-tricalcium phosphate and hydroxyapatite. J Eur Ceram Soc 2006; 26:1473 - 9; http://dx.doi.org/10.1016/j.jeurceramsoc.2005.02.007
- Yoshida Y, Van Meerbeek B, Snauwaert J, Hellemans L, Lambrechts P, Vanherle G, et al. A novel approach to AFM characterization of adhesive tooth-biomaterial interfaces. J Biomed Mater Res 1999; 47:85 - 90; http://dx.doi.org/10.1002/(SICI)1097-4636(199910)47:1<85::AID-JBM12>3.0.CO;2-H; PMID: 10400885
- Clark PA, Clark AM, Rodriguez A, Hussain MA, Mao JJ. Nanoscale characterization of bone-implant interface and biomechanical modulation of bone ingrowth. Mater Sci Eng C 2007; 27:382 - 93; http://dx.doi.org/10.1016/j.msec.2006.05.056
- Kaufmann EABE, Ducheyne P, Radin S, Bonnell DA, Composto R. Initial events at the bioactive glass surface in contact with protein-containing solutions. J Biomed Mater Res 2000; 52:825 - 30; http://dx.doi.org/10.1002/1097-4636(20001215)52:4<825::AID-JBM28>3.0.CO;2-M; PMID: 11033566
- Puleo DA, Nanci A. Understanding and controlling the bone-implant interface. Biomaterials 1999; 20:2311 - 21; http://dx.doi.org/10.1016/S0142-9612(99)00160-X; PMID: 10614937
- Ducheyne P, Qiu Q. Bioactive ceramics: the effect of surface reactivity on bone formation and bone cell function. Biomaterials 1999; 20:2287 - 303; http://dx.doi.org/10.1016/S0142-9612(99)00181-7; PMID: 10614935
- Jarcho M. Calcium phosphate ceramics as hard tissue prosthetics. Clin Orthop Relat Res 1981; 157:259 - 78; PMID: 7018783
- Daculsi G, LeGeros RZ, Mitre D. Crystal dissolution of biological and ceramic apatites. Calcif Tissue Int 1989; 45:95 - 103; http://dx.doi.org/10.1007/BF02561408; PMID: 2505900
- Kim H-M, Himeno T, Kokubo T, Nakamura T. Process and kinetics of bonelike apatite formation on sintered hydroxyapatite in a simulated body fluid. Biomaterials 2005; 26:4366 - 73; http://dx.doi.org/10.1016/j.biomaterials.2004.11.022; PMID: 15701365
- Dey A, Bomans PHH, Müller FA, Will J, Frederik PM, de With G, et al. The role of prenucleation clusters in surface-induced calcium phosphate crystallization. Nat Mater 2010; 9:1 - 5; http://dx.doi.org/10.1038/nmat2900; PMID: 20019657
- Neo M, Kotani S, Fujita Y, Nakamura T, Yamamuro T, Bando Y, et al. Differences in ceramic-bone interface between surface-active ceramics and resorbable ceramics: a study by scanning and transmission electron microscopy. J Biomed Mater Res 1992; 26:255 - 67; http://dx.doi.org/10.1002/jbm.820260210; PMID: 1569117
- Porter AE. Nanoscale characterization of the interface between bone and hydroxyapatite implants and the effect of silicon on bone apposition. Micron 2006; 37:681 - 8; http://dx.doi.org/10.1016/j.micron.2006.03.006; PMID: 16632368
- Porter AE, Hobbs LW, Rosen VB, Spector M. The ultrastructure of the plasma-sprayed hydroxyapatite-bone interface predisposing to bone bonding. Biomaterials 2002; 23:725 - 33; http://dx.doi.org/10.1016/S0142-9612(01)00177-6; PMID: 11771693
- Jones AC, Arns CH, Sheppard AP, Hutmacher DW, Milthorpe BK, Knackstedt MA. Assessment of bone ingrowth into porous biomaterials using MICRO-CT. Biomaterials 2007; 28:2491 - 504; http://dx.doi.org/10.1016/j.biomaterials.2007.01.046; PMID: 17335896
- Jones AC, Arns CH, Hutmacher DW, Milthorpe BK, Sheppard AP, Knackstedt MA. The correlation of pore morphology, interconnectivity and physical properties of 3D ceramic scaffolds with bone ingrowth. Biomaterials 2009; 30:1440 - 51; http://dx.doi.org/10.1016/j.biomaterials.2008.10.056; PMID: 19091398
- Malmström J, Adolfsson E, Arvidsson A, Thomsen P. Bone response inside free-form fabricated macroporous hydroxyapatite scaffolds with and without an open microporosity. Clin Implant Dent Relat Res 2007; 9:79 - 88; http://dx.doi.org/10.1111/j.1708-8208.2007.00031.x; PMID: 17535331
- Kitsugi T, Yamamuro T, Nakamura T, Oka M. Transmission electron microscopy observations at the interface of bone and four types of calcium phosphate ceramics with different calcium/phosphorus molar ratios. Biomaterials 1995; 16:1101 - 7; http://dx.doi.org/10.1016/0142-9612(95)98907-V; PMID: 8519932
- Daculsi G, LeGeros RZ, Heughebaert M, Barbieux I. Formation of carbonate-apatite crystals after implantation of calcium phosphate ceramics. Calcif Tissue Int 1990; 46:20 - 7; http://dx.doi.org/10.1007/BF02555820; PMID: 2153039
- de Bruijn JD, van Blitterswijk CA, Davies JE. Initial bone matrix formation at the hydroxyapatite interface in vivo. J Biomed Mater Res 1995; 29:89 - 99; http://dx.doi.org/10.1002/jbm.820290113; PMID: 7713963
- De Lange GL, De Putter C, De Wijs FLJA. Histological and ultrastructural appearance of the hydroxyapatite-bone interface. J Biomed Mater Res 1990; 24:829 - 45; http://dx.doi.org/10.1002/jbm.820240704; PMID: 2398074
- Hemmerle´ J, Cuisinier FJG, Schultz P, Voegel JC. HRTEM study of biological crystal growth mechanisms in the vicinity of implanted synthetic hydroxyapatite crystals. J Dent Res 1997; 76:682 - 7; http://dx.doi.org/10.1177/00220345970760020901; PMID: 9062562
- Xin R, Leng Y, Chen J, Zhang Q. A comparative study of calcium phosphate formation on bioceramics in vitro and in vivo. Biomaterials 2005; 26:6477 - 86; http://dx.doi.org/10.1016/j.biomaterials.2005.04.028; PMID: 15992923
- Leng Y, Chen J, Qu S. TEM study of calcium phosphate precipitation on HA/TCP ceramics. Biomaterials 2003; 24:2125 - 31; http://dx.doi.org/10.1016/S0142-9612(03)00036-X; PMID: 12699649
- Xin R, Leng Y, Wang N. Ultrastructure study of hydroxyapatite precipitation on ceramic surfaces in dog model. Mater Sci Eng C 2008; 28:1255 - 9; http://dx.doi.org/10.1016/j.msec.2007.11.006
- Fujita R, Yokoyama A, Nodasaka Y, Kohgo T, Kawasaki T. Ultrastructure of ceramic-bone interface using hydroxyapatite and β-tricalcium phosphate ceramics and replacement mechanism of β-tricalcium phosphate in bone. Tissue Cell 2003; 35:427 - 40; http://dx.doi.org/10.1016/S0040-8166(03)00067-3; PMID: 14580356
- Grandfield K, McNally EA, Palmquist A, Botton GA, Thomsen P, Engqvist H. Visualizing biointerfaces in three dimensions: electron tomography of the bone-hydroxyapatite interface. J R Soc Interface 2010; 7:1497 - 501; http://dx.doi.org/10.1098/rsif.2010.0213; PMID: 20534599
- Woodard JR, Hilldore AJ, Lan SK, Park CJ, Morgan AW, Eurell JAC, et al. The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials 2007; 28:45 - 54; http://dx.doi.org/10.1016/j.biomaterials.2006.08.021; PMID: 16963118
- Weiss P, Obadia L, Magne D, Bourges X, Rau C, Weitkamp T, et al. Synchrotron X-ray microtomography (on a micron scale) provides three-dimensional imaging representation of bone ingrowth in calcium phosphate biomaterials. Biomaterials 2003; 24:4591 - 601; http://dx.doi.org/10.1016/S0142-9612(03)00335-1; PMID: 12951002
- Schramm N, Hoppin J, Lackas C, Gershman B, Norenberg J, de Jong M. Improving resolution, sensitivity and applications for the NanoSPECT/CT: A high-performance SPECT/CT imager for small-animal research. J Nucl Med Meeting Abstracts 2007; 48:436