Abstract
The vascularization of new tissue within a reasonable time is a crucial prerequisite for the success of different cell- and material-based strategies. Considering that angiogenesis is a multi-step process involving humoral and cellular regulatory components, only in vivo assays provide the adequate information about vessel formation and the recruitment of endothelial cells. The present study aimed to investigate if neonatal human dermal fibroblasts could influence in vivo neovascularization. Results obtained showed that fibroblasts were able to recruit endothelial cells to vascularize the implanted matrix, which was further colonized by murine functional blood vessels after one week. The vessels exhibited higher levels of hemoglobin, compared with the control matrix, implanted without fibroblasts, in which no vessel formation could be observed. No significant differences were detected in systemic inflammation. The presence of vessels originated from the host vasculature suggested that host vascular response was involved, which constitutes a fundamental aspect in the process of neovascularization. Fibroblasts implanted within matrigel increased the presence of endothelial cells with positive staining for CD31 and for CD34 and the production of collagen influencing the angiogenic process and promoting the formation of microvessels. New strategies in tissue engineering could be delineated with improved angiogenesis using neonatal fibroblasts.
Introduction
Angiogenesis represents the process of formation of new blood vessels from the pre-existing microvasculature, this being of major significance in development, reproduction and repair.Citation1 The basic vascular network is formed by endothelial cells, through which the exchange of gases, growth factors, nutrients and elimination of metabolites between blood and tissue occur.Citation2 In fact, the diffusion distance of nutrients and oxygen out of blood vessels ranges from 100 to 200 μm.Citation3 In this context, the vascularization of new tissue within a reasonable time is a crucial prerequisite for the success of different cell- and material-based strategies in the context of regenerative medicine.Citation4 One of the challenges in tissue engineering has become the development of artificial extracellular matrices that promote the ingrowth of microvessels in the injured site, for instance through the delivery of growth factors.Citation5 Alternatively, endothelial cell delivery systems, including their cocultures, for example with osteoblasts or mesenchymal stem cells in the framework of bone regeneration strategies, have also been shown to constitute adequate strategies to promote the formation of capillary networks, even without further addition of growth factors, indicating that the interaction between these cell types assures the release of the required growth factors.Citation6,Citation7
In tissue engineering strategies, where the vascularization of implanted cell-scaffold constructs is a requirement, with a view to the use of a cell-scaffold construct, the interconnectivity between pores, in the case of porous scaffolds, or timely matrix degradation, are prerequisites for the success of both host cell invasion as well as proliferation and migration of implanted cells.Citation8-Citation11 Additionally, the vascular networks formed need to be guided by the implanted artificial matrix to promote anastomosis with the host vasculature.Citation12-Citation14 Matrigel has been extensively used as an artificial extracellular matrix to study angiogenesis in vitro and in vivo due to its unique characteristics. Matrigel is a mixture of extracellular matrix and basement membrane proteins extracted from the mouse Engelbreth-Holm-Swarm (EH) sarcoma that forms a hydrogel.Citation15 Considering that angiogenesis is a multi-step process, involving humoral and cellular regulatory components,Citation16 in vivo angiogenesis assays constitute the most informative experiments in terms of vessel formation, endothelial cell recruitment and vessel functionality through analysis of anastomosis with the host vasculature. Matrigel is especially suited for in vivo studies of angiogenesis due to its similarity with the natural extracellular matrix, despite containing a number of growth factors believed to play key roles in the process. In the attempt to reduce the influence of the growth factors in angiogenesis experiments a reduced growth factor matrigel (GFR-matrigel) was developed. The subcutaneous implantation of matrigel with mesenchymal progenitor cells into mice showed the capacity of these cells to induce angiogenesis, and thus it has been suggested as a model to develop approaches to improve neovascularization of engineered human tissues and organs.Citation14
The role of fibroblasts in tissue regeneration is probably underestimated in tissues other than the skin. Fibroblasts already constitute the dermis component of the commercial tissues engineered for dermal replacements used to treat chronic foot ulcers.Citation17 A cardiac patch using these cells has also been used to support the angiogenesis in infarcted heart tissue in animal models.Citation18,Citation19 Fibroblasts are known to maintain the structural integrity of connective tissues by continuously secreting growth factors and extracellular matrix precursors, which are essential for endothelial cell adhesion and spreading.Citation20 Furthermore, Kern et al. reported the lower immunogenicity of allogeneic fibroblasts grown in 3D compared with fibroblasts grown in monoculture.Citation21 In the present work, it was hypothesized that neonatal human dermal fibroblasts may influence the neovascularization of the GFR-matrigel. In order to provide information about the morphology of fibroblasts and how they could influence the neovascularization an in vivo GFR-matrigel plug assay was used. Hemoglobin content and inflammatory levels were measured and histological analyses were performed.
Results
Hemoglobin content
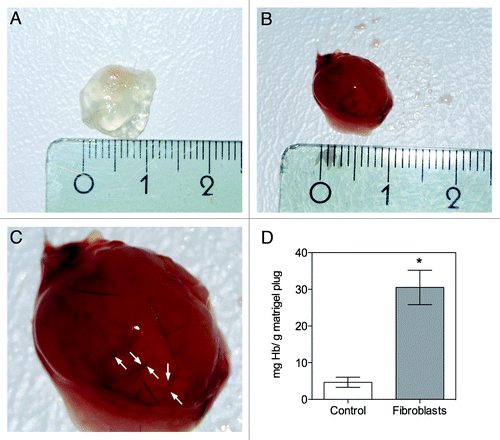
To evaluate if the implanted human neonatal fibroblasts could modulate the angiogenic process in vivo, and the recruitment of endothelial cells from the host in particular, the matrigel plug angiogenesis assay was used. Upon removal the plugs were easily distinguishable from the surrounding tissues, and a well-defined plug between skin and muscles could be excised for the preparation of histological sections. The extension of functional neovascularisation was evaluated by measuring the amount of hemoglobin (Hb) present in the matrigel plugs implanted with or without fibroblasts after one week. Matrigel implants which were inoculated with fibroblasts induced vascular development () contrary to what was observed in the control conditions, where no cells were implanted (). The plugs implanted with fibroblasts showed an angiogenic response, highlighted by the red color spread in the whole plug (; arrows suggest the presence of vessels in the matrigel plug implanted with fibroblasts). As can be seen in , the Hb levels were significantly increased in the matrigel plugs implanted with fibroblasts compared with the control group (without fibroblasts).
Figure 1. In vivo evaluation of angiogenesis using the matrigel plug assay. A mixture of matrigel and heparin, with or without fibroblasts (control), was implanted subcutaneously into C57BL/6J mice, and removed after one week. (A) Representative macroscopic visualization of matrigel plugs implanted without fibroblasts, which appear avascular; (B) macroscopic visualization of matrigel plugs implanted with fibroblasts; (C) magnification of image B, the arrows suggests the presence of blood vessels; (D) Quantification of hemoglobin (Hb) in the retrieved plugs, showing a significant increase in Hb levels upon fibroblast implantation. The presence of fibroblasts increased vessel formation in the plug, significantly differing from the control. Results are means ± SEM of independent experiments (n = 6). *p < 0.05 vs. control.
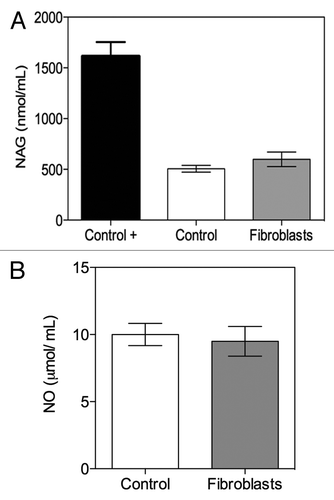
Inflammation-associated factor determination
To assess if implanted fibroblasts developed inflammation in mice during the experiment, N-acetylglucosaminidase (NAG) activity was evaluated in the serum of each animal. The results revealed no significant difference between the serum NAG activity levels of mice transplanted with fibroblasts compared with controls, indicating that no systemic inflammatory response to implanted fibroblasts could be detected (). Regarding the production of nitric oxide (NO), no significant differences between the two test groups were detected either (), thus confirming that no differences were apparent within these two groups.
Histological and immunohistological analysis
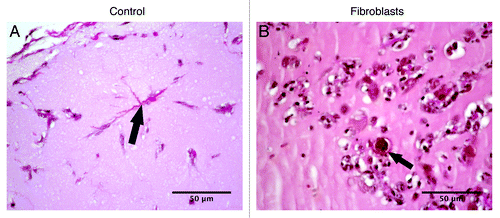
Histological analysis permits a microscopical view of the cell populations within the plugs in the two analyzed groups. All explanted plugs showed a slight inflammatory reaction with granulocytes and lymphocytes in the surrounding tissue after one week. Regarding the inflammatory reaction no differences could be found between the two analyzed groups. Furthermore, in all examined plugs an infiltration of cells could be found (). In matrigel with fibroblasts an accumulation of cells was observed not only at the border but also in the periphery and the center of the plug (). In this group, numerous areas of circularly arranged cells could be detected within the matrigel, which immunhistologically expressed CD31 (, arrows). In the specimens without fibroblasts capillary-like tubes (black arrow) could be found solely in the periphery of the plugs but not in the center (), which corresponds to the small amount of hemoglobin detected in the control group.
Figure 3. Representative images of hematoxylin and eosin staining of matrigel plugs implanted subcutaneously in C57BL/6J mice with (B and D) or without fibroblasts [control, (A and C)]. (B) Asterisk indicating population of cells in the border of the plug; (C) capillary-like tubes, arrow. (D) Circularly arranged cells, arrow.
![Figure 3. Representative images of hematoxylin and eosin staining of matrigel plugs implanted subcutaneously in C57BL/6J mice with (B and D) or without fibroblasts [control, (A and C)]. (B) Asterisk indicating population of cells in the border of the plug; (C) capillary-like tubes, arrow. (D) Circularly arranged cells, arrow.](/cms/asset/054acbde-6771-42d0-8f8b-f3c993f720b0/kbim_a_10920063_f0003.gif)
The in vivo recruitment of an angiogenic network into the matrigel plugs implanted with or without fibroblasts was also evaluated. In order to visualize the formation of new blood vessels in the implanted plugs, histological sections were stained with antibodies against CD31 and CD34 for endothelial cells. Neovascularization of the implanted matrigel plugs with fibroblasts was observed within one week after the injection. At this time it was possible to observe endothelial cells lining together and forming new blood vessels ( and ). The positive immunohistochemical staining for CD31 and CD34, both markers for endothelial cells (black arrow, and ), revealed the presence of microvessels (e.g., black circle, ). On the contrary, in the control group (), only few endothelial cells were noticeable, marked positively for CD31, that were not organized in vessel structures.
Figure 4. Cellular phenotypes colonizing matrigel plugs after one week of implantation. Representative images for CD31 staining: (A) matrigel implanted without fibroblasts (control) and (B) matrigel implanted with fibroblasts, arrow pointing out the CD31-positive cells and circular structures indicating the microvessels.
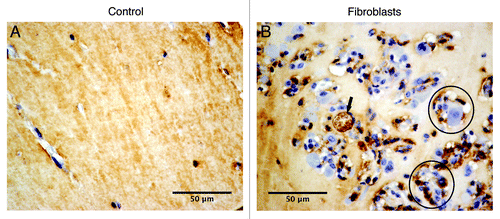
Figure 5. Cellular phenotypes colonizing matrigel plugs implanted with fibroblasts after one week of implantation. Representative images of stainings for CD31 and CD34: (A) microvessels formation, with arrows showing CD31 staining; (B) microvessels formation, with arrows indicating CD34 staining; (C and D) consecutives images [(C), CD31 staining; (D), CD34 staining], with arrows showing the vessels formed from the periphery to inside the plug.
![Figure 5. Cellular phenotypes colonizing matrigel plugs implanted with fibroblasts after one week of implantation. Representative images of stainings for CD31 and CD34: (A) microvessels formation, with arrows showing CD31 staining; (B) microvessels formation, with arrows indicating CD34 staining; (C and D) consecutives images [(C), CD31 staining; (D), CD34 staining], with arrows showing the vessels formed from the periphery to inside the plug.](/cms/asset/cda4b634-44eb-427e-88ad-fc1b10cd2a81/kbim_a_10920063_f0005.gif)
In matrigel plugs implanted with fibroblasts () it was also possible to observe endothelial, which collaborated in the process of formation of the blood vessels and stained positive for CD34 (). Near the periphery, it was observed vessel formation from the host vasculature staining positively for CD31 () and CD34 ().
To demonstrate the presence of fibrillar collagen and to understand if the cells during the migration into matrigel leave a track of collagen that could guide the endothelial cells during angiogenesis, the Elastic van Gieson staining was used. shows a track of collagen observed in the control samples (without fibroblasts). On the other hand, in the matrigel plugs implanted with fibroblasts () the collagen was observed in localized areas and around the microvessel (black arrow). This could be related with different time points of the vascularization of the matrigel.
Figure 6. Elastic van Gieson staining identifying fibers such as collagen (pink color). (A) Matrigel implanted without fibroblasts (control), with arrows pointing the track of collagen. (B) Matrigel implanted with fibroblasts, with arrow pointing out the collagen.
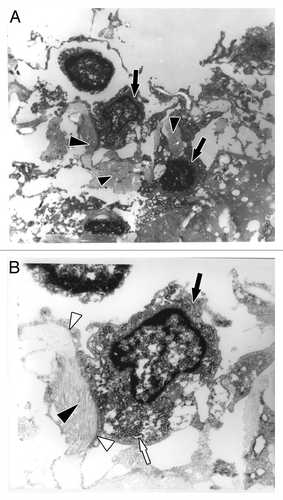
Then, in order to access if fibroblasts produced collagen in the matrigel plug, ultrastructural studies (TEM) were performed in the explants after one week. In fibroblasts are indicated by black arrows and the collagen produced by these cells are pointed out by black arrowheads. The high activity of protein synthesis that could be observed may be related with rough endoplasmic reticulum (RER, white arrow) presented over a large area of the cell (). It was possible to observe the well-organized collagen bundles (black arrowhead) surrounded by the filopodia extensions of the fibroblast (white arrowhead, ).
Figure 7. Ultrastructure of fibroblasts immobilized in a matrigel plug after one week of subcutaneous implantation by transmission electron microscopy. (A) The implanted fibroblasts produced collagen in the matrigel (black arrowhead indicates collagen; black arrows indicate fibroblasts); amplification, 6,500x. (B) Magnified view of a fibroblast (black arrow), well-developed rough endoplasmic reticulum (white arrow) indicating high protein synthesis, collagen (black arrowhead) surrounded by filopodia extensions of fibroblasts (white arrowhead); amplification, 17,500x.
Discussion
Understanding how to promote angiogenesis in a damaged tissue by using cells embedded in an injectable biomaterial continues to be a challenge in tissue regeneration. The need for proper vascularization, which involves the assembly of a microvascular network and its anastomosis with the host vasculature, remains one of the major hurdles for clinical success of complex tri-dimensional tissue engineered structures.Citation22 In order to address if neonatal human dermal fibroblasts could influence the formation of early microvascular structures the matrigel plug model was used. The matrigel plug assay has been described as a good model to study angiogenesis because it protects growth factors from degradation and dispersionCitation23,Citation24 and additionally provides the embedded cells with a suitable environment to proliferate and differentiate without scattering.Citation25,Citation26
The present in vivo study supports the hypothesis that implanted fibroblasts were able to recruit endothelial cells to vascularize an implanted artificial extracellular matrix with functional vessels after one week. As observed, the same matrix implanted without fibroblasts did not become vascularized, as confirmed by hemoglobin levels. Regarding the time-frame of one week, the cell infiltration and the observation of angiogenesis events in this period seems to be in agreement with the literature.Citation26,Citation27
Soluble factors produced by fibroblasts, such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF), probably influenced the interactions with endothelial cells and their recruitment. VEGF is required for survival, differentiation, and network formation of endothelial cells.Citation28 bFGF promotes both endothelial cell scattering, that is required during the first step of the angiogenesis process, and the formation of the cell-cell interactions required for vessel maturation.Citation23,Citation24,Citation29,Citation30 Presta et al. presented evidence suggesting the possibility that bFGF indirectly induces neovascularization by activation of the VEGF/VEGFR system.Citation29
Angiogenesis and inflammation are intimately related since immune cells produce cytokines and growth factors that target endothelial cells and stimulate angiogenesis and, in turn, the new vessels formed transport oxygen and nutrients, as well as immune cells.Citation31 In tissue regeneration it seems important to modulate inflammation to an adequate level.Citation32 Thus, it was evaluated if fibroblasts transplanted with matrigel changed the inflammatory profile by measuring inflammatory markers, namely NO and NAG. NAG is a lysosomal enzyme which shows increased activity in monocytes and macrophages activated during inflammatory diseases.Citation33 NO is related with general inflammation and is a vasodilator.Citation34 No significant differences were detected in systemic inflammation between matrigel implanted with or without fibroblasts. In this study human cells were used in animals that are not immunodeficient, which could further contribute to induce an immune response. However, the cell type used, namely human neonatal foreskin fibroblasts, do not express enough antigens to originate an enhanced immune response. Human neonatal foreskin cells are reported not to induce a significant immune response because the cells derived from neonatal human tissue have undeveloped HLA tissue markers.Citation21 Furthermore, fibroblasts from dermis are relatively nonantigenic and do not express HLA-DR markers.Citation21
In matrigel plugs implanted with fibroblasts the presence of vessels with endothelial cells, showing positive staining for CD31 and CD34 outlines different stages of vascularization. The presence of vessels originating from the host vasculature is a remarkable observation, showing that host vascular response was involved, which is a key step for the clinical success of vascularization strategies. These findings indicate that the environment created by fibroblasts favors the ingrowth of vessels from the host vasculature. Whether circulating endothelial progenitor cells (EPCs) contribute to the vascularization of the matrigel plugs in these experiments remains to be studied. EPCs are involved in promoting physiologic and pathologic neovascularization but questions remain about the magnitude of their contribution to newly forming blood vessels.Citation35 Melero-Martin et al. implanted EPCs in matrigel and reported no secretion of VEGF and no stimulation of murine vessel infiltration, although in cocultures of EPCs with mesenchymal stem cells better vascularization was observed.Citation25
Another key step in angiogenesis and vasculogenesis seems to be the production of new matrix. Mercier et al. reported that neurogenesis and vasculogenesis have implicated the participation of macrophages, fibroblasts and collagen.Citation36 Anghelina et al. reported that vascularization in a matrigel plug supplement with bFGF was influenced by initial cellular infiltration, with a predominant population of monocytes and macrophages.Citation26 The cells left a track forming cell columns after one week of implantation through secretion of angiogenic factors and enzymes able to degrade the matrix. A similar mechanism of cell invasion has been reported for leukocytes and tumor cells penetrating the matrix, or osteoclasts degrading and invading bone.Citation26 The amount of fibrillar collagen produced, apparently by fibroblasts, could derive from the circulating fibrocytes.Citation37 The present results showed the presence of collagen in matrigel plugs implanted with fibroblasts. By TEM, it was possible to observe the higher activity of the rough endoplasmic reticulum of fibroblasts, which is related with the production of collagen. Collagen acts as a potent pro-angiogenic milieu in itself,Citation38 whereas the matrix-associated fibroblasts might conceivably direct the orientation of new blood vessels.Citation39 In addition, the microenvironment present in the implant is very important for cell fate. For instance, Yin et al. reported that the extracellular microenvironment (soluble factors, cell-matrix or cell-cell interactions) could influence the chondrogenic differentiation of dermal fibroblasts,Citation40 although Reddi et al. reported that the transformation of fibroblasts to chondroblasts was unstable when compared with osteoblasts.Citation41 Moreover, concerning the ability of fibroblasts to synthesize collagen, Kellar et al. reported that dermal fibroblasts cultured in 3D structures could be used as a support or delivery system of cells, like stem cell populations in cardiac diseases.Citation42 Dermal fibroblasts have also been described as a population of cells with mesenchymal origin having a crucial role in communication with other cell types in response to contextual signals in skin physiology.Citation43 The present strategy revealed that an adequate environment stimulating collagen production by transplanted fibroblasts could contribute to the ingrowth of vessels from the host vasculature.
The matrigel plug assay is accepted as an adequate model for in vivo studies of angiogenesis and vasculogenesis. Anghelina et al. suggested the use of matrigel plugs as experimental models of tissue regeneration, in which neovascularization is related with fibrosis and organogenesis and where inflammatory cells play a key structural role.Citation26 However, matrigel it is not approved for use in humans. Taking this into account, new hydrogel systems need to be designed, which provide an adequate microenvironment to mimic the natural extracellular matrix, with its capability to regulate the signaling properties of soluble and insoluble components of the matrix. Alternative biofunctional hydrogels have been shown to provide adequate in vivo models of tumor angiogenesis.Citation44 New and complex biofunctional hydrogel matrices based on alginate have been recently developed, mimicking the extracellular matrix in its cell-specific adhesion and susceptibility to cell-driven proteolysis.Citation9 Other hydrogel-based systems have been reported as promising injectable artificial extracellular matrices for cell transplantation and delivery.Citation9,Citation10,Citation45-Citation49
Conclusions
The present in vivo study supports the hypothesis that fibroblasts embedded in matrigel plugs implanted in mice are able to synthesize collagen and promote the ingrowth of vessels from the host vasculature. In the absence of any marked acute inflammation the current strategy provides an adequate microenvironment for new strategies in tissue engineering, where neovascularization is essential.
Materials and Methods
Culture of fibroblasts
Neonatal human dermal foreskin fibroblasts-1 (from American Type Culture Collection, ATTC) were cultured in Dulbecco's modified Eagle's medium (DMEM, Gibco) with high glucose and L-glutamine, supplemented with 15% v/v inactivated fetal bovine serum (FBS, from Gibco), 100 U/mL penicillin G and 100 μg/mL streptomycin (Gibco). The cells were maintained at 37°C in a humidified 5% CO2 atmosphere and they were cultured until 90% confluence was reached. Fibroblasts were used at passages 6–8 in every experiment and the culture medium was changed every two days.
In vivo angiogenesis assay
Animal experiments were conducted according to accepted standards of human animal care, namely European Community guidelines (86/609/EEC) and Portuguese Act (129/92). In vivo matrigel plug assay was performed using a mixture of growth factor-reduced Matrigel® (GFR-Matrigel®, BD Science) and heparin (25 U/mL, Sigma) with 106 fibroblasts. Five hundred microliters of the GFR-Matrigel® mixture were subcutaneously inoculated into eight-week-old C57BL/6J male mice, from Charles River, (n = 7). A control group was used, without cells (n = 7). After one week mice were anesthetized by intraperitoneal injection of pentobarbital sodium (200 mg/kg body weight) and euthanized, the matrigel plugs were removed, weighed, photographed and fixed. Mouse blood was also collected for evaluation of inflammatory markers (N-acetylglucosaminidase activity and nitric oxide production). The formation of the functional vasculature in the matrigel plug was evaluated by determination of the hemoglobin concentration in the homogenized plug.
Determination of hemoglobin
Hemoglobin (Hb) content was determined in matrigel plugs collected one week after implantation, using a previously described procedure.Citation49 Matrigel plugs were homogenized in a water-heparin solution and centrifugated at 1,500 g for 15 min at 20°C. The Hb content was measured in the supernatant (100 μL) according to the Drabkin’s method (Sigma) at 540 nm.
Determination of N-acetylglucosaminidase activity
Systemic inflammation can be ascertained by measuring the activity of the lysosomal enzyme N-acetylglucosaminidase (NAG) in the serum, an enzyme present in high levels in activated macrophages. The blood was collected and allowed to clot at room temperature for 30 min, followed by centrifugation at 1,500 g for 15 min at 25°C in order to obtain the serum. The serum was incubated for 10 min at 37°C with 100 µL of p-nitrophenyl-N-acetyl-β-D-glucosaminide solution in a 96-well plate. The reaction was stopped by the addition of 0.2 M glycine buffer (pH 10.6) and the substrate hydrolysis was measured at 405 nm.Citation49 As a positive control a mixture of matrigel and heparin with vascular endothelial growth factor (VEGF, 0.1 µg/mL) was used.
Determination of nitric oxide
Nitric oxide (NO) production was measured indirectly using the Griess Reaction system, which uses 1% sulphanylamide and 0.1% N-1-napthylethylenediamine dihydrochloride under 2.5% phosphoric acid to measure nitrite (NO2-) in serum. One hundered μL of serum and 100 μL of Griess Reagent were added to a 96-well plate. After 15 min at room temperature, protected from light, the azo product was measured at 550 nm.
Histological and immunohistological analysis
The explanted matrigel plugs were fixed in buffered formalin solution (10%), dehydrated using a graded ethanol series and automatically embedded in paraffin according to standard procedures. Paraffin sections of 4 μm thickness were cut, rehydrated and stained with hematoxylin and eosin staining according to standard procedures. To identify fibers secreted by fibroblasts the Elastica van Gieson (EvG) staining was performed according to laboratory standard methods.
The immunohistological detection of CD31 and CD34 was performed with the peroxidase method by using DAB as chromogen. For this purpose monoclonal antibodies against CD31 (dilution 1:400, Santa Cruz) and CD34 (dilution 1:200, Santa Cruz) were used. The immunohistological staining was automatically performed with help of an Autostainer plus (Dako) and the appropriate Dako-Staining kits. After the immunodetection the nuclei of cells were stained by hemalaun.
Transmission electron microscopy (TEM)
For transmission electron microscopical analysis samples of 0.5 × 0.5 cm were cut from the explants, fixed in buffered glutaraldehyde (2.5%) overnight, contrasted with OsO4 and then embedded in Agar 100 (Plano), which polymerized for at least 24 h. After trimming first semi-thin sections were performed to identify the region of interest, which was then cut as ultrathin sections with the Ultracut E microtome (Leica). Transmission electron microscopical analysis was performed with a Phillips EM 410 (Phillips). Visualization took place at 80 KV and images were photodocumented.
Statistical analysis
All experiments were performed at least in triplicate. Quantifications are expressed as mean ± standard error of the mean (SEM). For comparison between two groups the Student's t-test was used. A difference between experimental groups was considered significant with a confidence interval of 95%, whenever p < 0.05.
Acknowledgments
The work performed at INEB was financed by FEDER funds through the Programa Operacional Factores de Competitividade—COMPETE and by Portuguese funds through FCT—Fundação para a Ciência e a Tecnologia in the framework of projects PEST-C/SAU/LA0002/2011 and POCI/SAU-BMA/55556/2004 and the PhD scholarship SFRH/BD/40421/2007 for S.G.G.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Folkman J. Angiogenesis: an organizing principle for drug discovery?. Nat Rev Drug Discov 2007; 6:273 - 86; http://dx.doi.org/10.1038/nrd2115; PMID: 17396134
- Jain RK, Au P, Tam J, Duda DG, Fukumura D. Engineering vascularized tissue. Nat Biotechnol 2005; 23:821 - 3; http://dx.doi.org/10.1038/nbt0705-821; PMID: 16003365
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature 2000; 407:249 - 57; http://dx.doi.org/10.1038/35025220; PMID: 11001068
- Soker S, Machado M, Atala A. Systems for therapeutic angiogenesis in tissue engineering. World J Urol 2000; 18:10 - 8; http://dx.doi.org/10.1007/PL00007070; PMID: 10766038
- Kaigler D, Wang Z, Horger K, Mooney DJ, Krebsbach PH. VEGF scaffolds enhance angiogenesis and bone regeneration in irradiated osseous defects. J Bone Miner Res 2006; 21:735 - 44; http://dx.doi.org/10.1359/jbmr.060120; PMID: 16734388
- Bidarra SJ, Barrias CC, Barbosa MA, Soares R, Ame´de´e J, Granja PL. Phenotypic and proliferative modulation of human mesenchymal stem cells via crosstalk with endothelial cells. Stem Cell Res 2011; 7:186 - 97; http://dx.doi.org/10.1016/j.scr.2011.05.006; PMID: 21907162
- Unger RE, Sartoris A, Peters K, Motta A, Migliaresi C, Kunkel M, et al. Tissue-like self-assembly in cocultures of endothelial cells and osteoblasts and the formation of microcapillary-like structures on three-dimensional porous biomaterials. Biomaterials 2007; 28:3965 - 76; http://dx.doi.org/10.1016/j.biomaterials.2007.05.032; PMID: 17582491
- Yang S, Leong KF, Du Z, Chua CK. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng 2001; 7:679 - 89; http://dx.doi.org/10.1089/107632701753337645; PMID: 11749726
- Fonseca KB, Bidarra SJ, Oliveira MJ, Granja PL, Barrias CC. Molecularly designed alginate hydrogels susceptible to local proteolysis as three-dimensional cellular microenvironments. Acta Biomater 2011; 7:1674 - 82; http://dx.doi.org/10.1016/j.actbio.2010.12.029; PMID: 21193068
- Bidarra SJ, Barrias CC, Fonseca KB, Barbosa MA, Soares RA, Granja PL. Injectable in situ crosslinkable RGD-modified alginate matrix for endothelial cells delivery. Biomaterials 2011; 32:7897 - 904; http://dx.doi.org/10.1016/j.biomaterials.2011.07.013; PMID: 21784515
- Vacharathit V, Silva EA, Mooney DJ. Viability and functionality of cells delivered from peptide conjugated scaffolds. Biomaterials 2011; 32:3721 - 8; http://dx.doi.org/10.1016/j.biomaterials.2010.12.048; PMID: 21334064
- Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol 2005; 23:47 - 55; http://dx.doi.org/10.1038/nbt1055; PMID: 15637621
- Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature 2009; 462:433 - 41; http://dx.doi.org/10.1038/nature08602; PMID: 19940913
- Melero-Martin JM, Khan ZA, Picard A, Wu X, Paruchuri S, Bischoff J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood 2007; 109:4761 - 8; http://dx.doi.org/10.1182/blood-2006-12-062471; PMID: 17327403
- Auerbach R, Lewis R, Shinners B, Kubai L, Akhtar N. Angiogenesis assays: a critical overview. Clin Chem 2003; 49:32 - 40; http://dx.doi.org/10.1373/49.1.32; PMID: 12507958
- van der Bilt JD, Borel Rinkes IH. Surgery and angiogenesis. Biochim Biophys Acta 2004; 1654:95 - 104; PMID: 14984770
- Edmonds M, European and Australian Apligraf Diabetic Foot Ulcer Study Group. Apligraf in the treatment of neuropathic diabetic foot ulcers. Int J Low Extrem Wounds 2009; 8:11 - 8; http://dx.doi.org/10.1177/1534734609331597; PMID: 19189997
- Kellar RS, Landeen LK, Shepherd BR, Naughton GK, Ratcliffe A, Williams SK. Scaffold-based three-dimensional human fibroblast culture provides a structural matrix that supports angiogenesis in infarcted heart tissue. Circulation 2001; 104:2063 - 8; http://dx.doi.org/10.1161/hc4201.097192; PMID: 11673347
- Kellar RS, Shepherd BR, Larson DF, Naughton GK, Williams SK. Cardiac patch constructed from human fibroblasts attenuates reduction in cardiac function after acute infarct. Tissue Eng 2005; 11:1678 - 87; http://dx.doi.org/10.1089/ten.2005.11.1678; PMID: 16411813
- Mansbridge JN, Liu K, Pinney RE, Patch R, Ratcliffe A, Naughton GK. Growth factors secreted by fibroblasts: role in healing diabetic foot ulcers. Diabetes Obes Metab 1999; 1:265 - 79; http://dx.doi.org/10.1046/j.1463-1326.1999.00032.x; PMID: 11225638
- Kern A, Liu K, Mansbridge J. Modification of fibroblast gamma-interferon responses by extracellular matrix. J Invest Dermatol 2001; 117:112 - 8; http://dx.doi.org/10.1046/j.0022-202x.2001.01386.x; PMID: 11442757
- Choong CS, Hutmacher DW, Triffitt JT. Co-culture of bone marrow fibroblasts and endothelial cells on modified polycaprolactone substrates for enhanced potentials in bone tissue engineering. Tissue Eng 2006; 12:2521 - 31; http://dx.doi.org/10.1089/ten.2006.12.2521; PMID: 16995785
- Kawaguchi N, Toriyama K, Nicodemou-Lena E, Inou K, Torii S, Kitagawa Y. De novo adipogenesis in mice at the site of injection of basement membrane and basic fibroblast growth factor. Proc Natl Acad Sci U S A 1998; 95:1062 - 6; http://dx.doi.org/10.1073/pnas.95.3.1062; PMID: 9448285
- Passaniti A. Extracellular matrix-cell interactions: Matrigel and complex cellular pattern formation. Laboratory investigation; a journal of technical methods and pathology 1992; 67:804
- Melero-Martin JM, De Obaldia ME, Kang SY, Khan ZA, Yuan L, Oettgen P, et al. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ Res 2008; 103:194 - 202; http://dx.doi.org/10.1161/CIRCRESAHA.108.178590; PMID: 18556575
- Anghelina M, Krishnan P, Moldovan L, Moldovan NI. Monocytes/macrophages cooperate with progenitor cells during neovascularization and tissue repair: conversion of cell columns into fibrovascular bundles. Am J Pathol 2006; 168:529 - 41; http://dx.doi.org/10.2353/ajpath.2006.050255; PMID: 16436667
- Negrão R, Costa R, Duarte D, Taveira Gomes T, Mendanha M, Moura L, et al. Angiogenesis and inflammation signaling are targets of beer polyphenols on vascular cells. J Cell Biochem 2010; 111:1270 - 9; http://dx.doi.org/10.1002/jcb.22850; PMID: 20803553
- Kunz-Schughart LA, Schroeder JA, Wondrak M, van Rey F, Lehle K, Hofstaedter F, et al. Potential of fibroblasts to regulate the formation of three-dimensional vessel-like structures from endothelial cells in vitro. Am J Physiol Cell Physiol 2006; 290:C1385 - 98; http://dx.doi.org/10.1152/ajpcell.00248.2005; PMID: 16601149
- Underwood PA, Bean PA, Gamble JR. Rate of endothelial expansion is controlled by cell:cell adhesion. Int J Biochem Cell Biol 2002; 34:55 - 69; http://dx.doi.org/10.1016/S1357-2725(01)00100-5; PMID: 11733185
- Presta M, Dell’Era P, Mitola S, Moroni E, Ronca R, Rusnati M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev 2005; 16:159 - 78; http://dx.doi.org/10.1016/j.cytogfr.2005.01.004; PMID: 15863032
- Costa C, Incio J, Soares R. Angiogenesis and chronic inflammation: cause or consequence?. Angiogenesis 2007; 10:149 - 66; http://dx.doi.org/10.1007/s10456-007-9074-0; PMID: 17457680
- Almeida CR, Vasconcelos DP, Goncalves RM, Barbosa MA. Enhanced mesenchymal stromal cell recruitment via natural killer cells by incorporation of inflammatory signals in biomaterials. J R Soc Interface 2012; 9:261 - 71; PMID: 21752807
- Karnovsky ML, Lazdins JK. Biochemical criteria for activated macrophages. J Immunol 1978; 121:809 - 13; PMID: 357654
- Berrazueta JR, Lo´pez-Jaramillo P, Moncada S. [Nitric oxide: from endogenous vasodilator to biologic mediator]. Rev Esp Cardiol 1990; 43:421 - 31; PMID: 2093954
- Krenning G, van Luyn MJ, Harmsen MC. Endothelial progenitor cell-based neovascularization: implications for therapy. Trends Mol Med 2009; 15:180 - 9; http://dx.doi.org/10.1016/j.molmed.2009.02.001; PMID: 19303359
- Mercier F, Kitasako JT, Hatton GI. Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J Comp Neurol 2002; 451:170 - 88; http://dx.doi.org/10.1002/cne.10342; PMID: 12209835
- Mori L, Bellini A, Stacey MA, Schmidt M, Mattoli S. Fibrocytes contribute to the myofibroblast population in wounded skin and originate from the bone marrow. Exp Cell Res 2005; 304:81 - 90; http://dx.doi.org/10.1016/j.yexcr.2004.11.011; PMID: 15707576
- Abramovitch R, Dafni H, Neeman M, Nagler A, Pines M. Inhibition of neovascularization and tumor growth, and facilitation of wound repair, by halofuginone, an inhibitor of collagen type I synthesis. Neoplasia 1999; 1:321 - 9; http://dx.doi.org/10.1038/sj.neo.7900043; PMID: 10935487
- Martin TA, Harding KG, Jiang WG. Regulation of angiogenesis and endothelial cell motility by matrix-bound fibroblasts. Angiogenesis 1999; 3:69 - 76; http://dx.doi.org/10.1023/A:1009004212357; PMID: 14517446
- Yin S, Cen L, Wang C, Zhao G, Sun J, Liu W, et al. Chondrogenic transdifferentiation of human dermal fibroblasts stimulated with cartilage-derived morphogenetic protein 1. Tissue Eng Part A 2010; 16:1633 - 43; http://dx.doi.org/10.1089/ten.tea.2009.0570; PMID: 19995150
- Reddi AH, Huggins C. Biochemical sequences in the transformation of normal fibroblasts in adolescent rats. Proc Natl Acad Sci U S A 1972; 69:1601 - 5; http://dx.doi.org/10.1073/pnas.69.6.1601; PMID: 4504376
- Kellar RS, Williams SK, Naughton GK, Figliozzi GM, Siani-Rose M. Three-dimensional fibroblast cultures stimulate improved ventricular performance in chronically ischemic canine hearts. Tissue Eng Part A 2011; 17:2177 - 86; http://dx.doi.org/10.1089/ten.tea.2010.0680; PMID: 21529261
- Sorrell JM, Caplan AI. Fibroblast heterogeneity: more than skin deep. J Cell Sci 2004; 117:667 - 75; http://dx.doi.org/10.1242/jcs.01005; PMID: 14754903
- Hoffmann J, Schirner M, Menrad A, Schneider MR. A highly sensitive model for quantification of in vivo tumor angiogenesis induced by alginate-encapsulated tumor cells. Cancer Res 1997; 57:3847 - 51; PMID: 9288798
- Salgado CL, Sanchez EM, Zavaglia CA, Almeida AB, Granja PL. Injectable biodegradable polycaprolactone-sebacic acid gels for bone tissue engineering. Tissue Eng Part A 2012; 18:137 - 46; http://dx.doi.org/10.1089/ten.tea.2011.0294; PMID: 21902607
- Munarin F, Guerreiro SG, Grellier MA, Tanzi MC, Barbosa MA, Petrini P, et al. Pectin-based injectable biomaterials for bone tissue engineering. Biomacromolecules 2011; 12:568 - 77; http://dx.doi.org/10.1021/bm101110x; PMID: 21302960
- Sun Q, Silva EA, Wang A, Fritton JC, Mooney DJ, Schaffler MB, et al. Sustained release of multiple growth factors from injectable polymeric system as a novel therapeutic approach towards angiogenesis. Pharm Res 2010; 27:264 - 71; http://dx.doi.org/10.1007/s11095-009-0014-0; PMID: 19953308
- Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science 2009; 324:1673 - 7; http://dx.doi.org/10.1126/science.1171643; PMID: 19556500
- Bidarra SJ, Barrias CC, Barbosa MA, Soares R, Granja PL. Immobilization of human mesenchymal stem cells within RGD-grafted alginate microspheres and assessment of their angiogenic potential. Biomacromolecules 2010; 11:1956 - 64; http://dx.doi.org/10.1021/bm100264a; PMID: 20690708