Abstract
Adoptive transfer of stem cells has shown potential as an effective treatment for acute kidney injury (AKI). The current strategy for adoptive transfer of stem cells is by intravenous injection. However, this conventional method of stem cell delivery is riddled with problems causing reduced efficacy of the therapeutic potential of delivered stem cells. This review summarizes the recent advancements in an alternative method of stem cell delivery for treatment of AKI, embedding stem cells in hyaluronic acid (HA-) based hydrogels followed by their implantation. Furthermore, one stem cell type in particular, endothelial progenitor cells (EPC), have shown remarkable therapeutic benefits for treatment of AKI when delivered by HA-hydrogels. The review also summarizes the delivery of EPC by HA-hydrogels in the setting of AKI.
Stem Cells for Therapeutic Use
While the potential of stem cells for tissue repair and regeneration in treatment of disease and injury has been subject to intense investigation over the past 20 years, the full therapeutic potential of these cells have yet to be fully realized. During this time, various stem cell lines have been isolated and characterized including embryonic and adult stem cells such as hematopoietic, mesenchymal, cardiac, neuronal and retinal. All these various stem cell lines have been examined for their therapeutic benefits including their adoptive transfer for treatment of injuries and diseases as diverse as post-chemotherapy blood disorders, myocardial infarction, burns, spinal cord and brain injuries, eye injury, diabetes, Crohn disease and muscular dystrophy, to name a few.Citation1 The kidney has been no stranger to this ever expanding and evolving field. Stem cells have been demonstrated to possess a notable repair and regenerative potential when delivered to the injured kidney.Citation2-Citation8 However, currently much more advancement is needed before stem cell therapy is successfully applied clinically for broad scale use in the treatment of kidney disease.
One type of stem cells that has shown remarkable renoprotective potential without significant side effects are endothelial progenitor cells (EPC). EPC have been shown to improve renal function, attenuate the pro-inflammatory response associated with renal injury, and improve damage to tubules and renal vascular segments during kidney injury while providing enhanced neoangiogenesis.Citation2,Citation4,Citation7,Citation8 The beneficial attributes associated with EPC delivery for treatment of kidney damage counter the vascular impairment that occurs in the course of various episodes of acute kidney injury (AKI) that leads to the progressive nature of renal dysfunction and disease.Citation2,Citation7-Citation9 An intact and healthy EPC niche, residing in the bone marrow but also found locally in renal vascular beds such as in the area of the adventitia layer of vessels, is relied on to maintain normal vascular function including maintenance and possible replacement of the endothelium.Citation10-Citation12 The loss of EPC integrity during kidney disease was illustrated by our group in an Adriamycin model of nephropathy,Citation8 in which the progressive nature of renal injury was heavily influenced by the destruction of competent endogenous EPC. The deterioration of the bone marrow EPC niche prevented both the mobilization of these cells to the sites of renal injury and ensuing repair of damage. When exogenous EPC were adoptively transferred, renal function considerably improved. These results are not exclusive to Adriamycin-induced nephropathy, but have also been seen in other models of AKI such as sepsis-induced AKI.Citation2
Problems with Current Methods of Cell Therapy: Treatment of AKI
One of the major problems confronting current stem cell (including EPC) therapy is a method for cell delivery. When stem cells are delivered by IV injection, less than 3% of the delivered cells find their way to the injured kidney and engraft, while majority of delivered cells undergo programmed cell death (anoikis) before they are capable of providing any therapeutic benefits to damaged tissues.Citation13 Many current trials examining the delivery of stem cells for treatment of kidney disease use IV injection of large quantities of cells (usually around 1 million cells per injection), administered all at once into the circulation by a bolus IV injection. Often times these delivered cells become trapped in the pulmonary vasculature causing embolism or suffer from anoikis before ever making to the injured kidneys.Citation13 Furthermore, if the impairment of kidney function is due to circulating factors such as cytotoxins, then stem cells introduced into the circulation by IV injection become susceptible to the harmful effects of such circulating toxins.
Another major problem of IV delivery of stem cells is related to integrin dependent activation and homing of delivered cells. While β2 integrins are the major regulators of EPC transendothelial migration, integrins α5β1, α6β1, αvβ3 and αvβ5 are major determinants of EPC homing, invasion, differentiation and paracrine factor production with integrin α4β1 being a key regulator of EPC retention and/or mobilization from the bone marrow.Citation14 The expression and activation of these integrins on the surface of stem cells is critical for their homing to proper sites of damage, adherence and exertion of their renoprotective paracrine influence.Citation14 In essence, integrins give EPC a “guidance mechanism” to find their way around the circulation and locate the targeted damaged tissue. Furthermore, activation of integrins on the surface of EPC, and subsequent effects on intracellular cytoskeleton properties within the EPC, has also been shown to enhance the release of both anti-inflammatory and pro-regenerative substancesCitation14 from EPC enhancing their reparative capabilities. Integrins are activated once these cells bind to substrates that contain a RGD sequence (an arginine-glycine-aspartic acid conserved motif found in cell adhesion substrates such as fibronectin).Citation15,Citation16 When EPC are delivered by IV injection, because of the aqueous nature of both the delivery vehicle solution and the circulating plasma, delivered cells lack substrate RGD adherence cues that would trigger the expression and activation of integrins on their surface. The lack of integrin activation on delivered EPC prevents these cells from fully eliciting their protective effects. This presents yet another problem with conventional methods of IV delivery of cells.
Bioengineered Scaffolds for Stem Cell Delivery: Treatment of AKI
In attempt to protect delivered stem cells and to provide them with a microenvironment conducive to their viability while maximizing their therapeutic potential, our laboratory has experimented with the delivery of EPC embedded in bioengineered scaffolds. Scaffolds provide substantial flexibility because they can be constructed from many different substances and their properties chemically changed to allow for creation of alternative niche-like microenvironments. For instance, variable scaffold compositions can be manipulated to affect embedded stem cell retention, differentiation and proliferation while also influencing the release of anti-inflammatory molecules from embedded cells.Citation17-Citation19 The selection of specific biomaterial compositions for scaffold engineering can provide embedded cells with surroundings resembling the endogenous extracellular matrixCitation20 and offer many advantages in regard to cell therapy applications. The use of such biomaterials allows for direct delivery and retention of EPC at the precise location of tissue damage leading to the improved delivery efficiency, a more intense therapeutic response, and avoidance of side effects related to systemic IV delivery.
Various bioengineered scaffolds have been constructed and tested for cell therapy purposes. For embedding of stem cells, it is highly desirable to create scaffolds that closely mimic the endogenous stem cell niche. Collagen, gelatin, fibrin, silk, agarose, alginate, dextran, cellulose, chitosan, heparin, chondroitin sulfate and hyaluronic acid engineered scaffolds are the more commonly and successfully used natural biomaterials for stem cell delivery in animal models.Citation21-Citation23 Recently, hyaluronic acid (HA) based hydrogels have emerged as a bioengineered scaffold that holds potential for its application in cell therapy. An advantage of stem cell delivery by embedding in HA-hydrogels is the ease of delivery. HA-hydrogels are implanted before their solidification, which allows for hydrogels with embedded cells to be delivered by a simple syringe injection. Once injected, the hydrogel concludes the solidification process within a few minutes. The delivery of HA-hydrogel scaffolds (without embedded cells) composed of thiol-modified gelatin (derived from collagen I and IV) results in the least inflammatory response, as compared with various other bioengineered scaffolds.Citation24 In experiments by our lab, we observed greatest response from embedded EPC when pronectin (50mg/ml) was added to these denatured collagen HA-hydrogels and crosslinked at 4% with polyethylene (glycol) diacrylate (PEGDA).Citation7 Addition of pronectin introduced the critical RGD binding element required by EPC for adhesion and activation of cell surface integrins including α5β1, α6β1, αvβ3, αvβ5 and α4β1.Citation14 The use of 4% PEGDA crosslinker provides optimal hydrogel rigidity and permits significant cell retention and viability while preventing infiltration of toxins present in the external milieu.Citation7
An advantage of HA-hydrogels is their ability to be readily dissolved on demand. Since the main constituents of hydrogels are denatured collagen and HA, these scaffolds can easily and rapidly be dissolved with hyaluronidase and collagenase, subsequently allowing the release and mobilization of embedded stem cells. Used in the correct concentrations, these digesting enzymes do not cause damage or phenotypical change to embedded cells.Citation7 The application of digesting enzymes is simple and consists of a mere injection directly into the implanted HA-hydrogel, followed by dissolution of the gel and complete mobilization of embedded cells within 24–48 h.Citation7 The ability to release embedded EPC on demand provides greater flexibility of cell therapy. For instance, HA-hydrogels with embedded EPC can be implanted into desired tissues where they remain until the release of EPC is required for local tissue repair. Meanwhile, the considerable retention of EPC in HA-hydrogels means embedded cells will stay in their implant locality (usually up to two weeks), while retaining their capability to secrete anti-inflammatory and pro-angiogenic molecules that can escape the scaffolding into the surrounding tissue and circulation.Citation25-Citation27 The ability to regulate the release of EPC from implanted scaffolds also allows flexibility in the timing of hydrogel implantation for treatment of kidney injury. The kidney is especially useful for HA-hydrogel delivery of stem cells because during kidney injury, the injured tissue endogenously releases hyaluronidase.Citation28,Citation29 The intrinsic release of hyaluronidase means the kidney, when injured, will automatically digest implanted HA-hydrogels even without injection of enzymes, allowing release of embedded stem cells. Since hyaluronidase is released when kidneys are initially injured, the digestion of HA-hydrogels and subsequent EPC release occurs at an optimal time during kidney injury allowing for EPC therapeutic effects to engage before damage becomes too extensive and irreparable. A schematic summary of the implantation of HA-hydrogels and subsequent release and mobilization of embedded EPC for treatment of AKI is provided in .
Figure 1. Schematic of the implantation of HA-hydrogels and subsequent release and mobilization of embedded EPC for treatment of AKI in a mouse model. 1) HA-hydrogels with embedded EPC are implanted either superficially into ears or subcapsularly into kidneys. 2) Induction of AKI (cyto-/endotoxins). 3a) Kidney implants are digested by endogenous release of hyaluronidase from the kidneys during AKI and embedded EPC are mobilized into the kidney, or 3b) ear implants are digested by direct injection of hydrogel-digesting enzymes and embedded EPC are mobilized into the circulation. 4) Released EPC generate therapeutic effects (see and ).
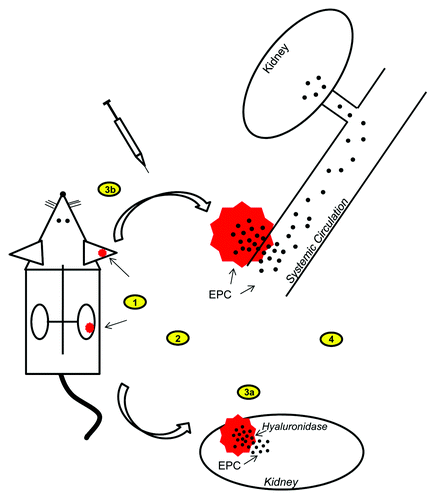
In studies by our lab, we have conducted multiple experiments using HA-hydrogels as a bioengineered scaffold for delivery of stem cells (EPC) for the treatment of various forms of AKI (the results of these experiments are summarized in and ).Citation2,Citation7 The first AKI model we examined was a model of Adriamycin (doxorubicin) induced nephropathy, which is characterized by tubulointerstitial damage, glomerulosclerosis with accompanying glomerular visceral epithelial cell damage and proteinuria.Citation30 In preliminary in vitro experiments, we first assessed if HA-hydrogel scaffolds offered protection to embedded EPC against toxins such as Adriamycin. Indeed, at concentrations of Adriamycin (1–50 uM) that normally caused cell damage and death, HA-hydrogel embedding protected cells against cytotoxicity and resulted in 6-fold enhanced cell viability of EPC.Citation7 This effect was primarily the result of the impediment for toxins to readily infiltrate the HA-hydrogel and damage embedded EPC. In a murine in vivo model, HA-hydrogels with embedded EPC were delivered to Adriamycin-induced AKI mice (ref). In these in vivo experiments, HA-hydrogels were implanted at two locations, subcutaneously in the ear (ease of microscopic monitoring of fluorescently labeled embedded cells) and supcapsularly in the kidney. While HA-hydrogels were implanted prior to Adriamycin-induced AKI, EPC were not released from these scaffolds until after administration of Adriamycin. Embedded EPC were released into the systemic circulation from ear implanted HA-hydrogels by direct injection of digestive enzymes within 1–2 h after Adriamycin administration. HA-hydrogels implanted in the kidney were digested by endogenous renally secreted hyaluronidase allowing for release of embedded EPC locally into the injured kidney. Adoptive transfer of EPC into mice with Adriamycin-induced AKI reduced both short- and long-term elevation in serum creatinine levels by 50–60% and long-term proteinuria by 60–75%, with greater improvement observed with EPC delivery by HA-hydrogel as compared with IV injection.Citation7 Engraftment of EPC into the damaged kidney was enhanced by as much as 6-fold when EPC were delivered by HA-hydrogel as opposed to IV delivery.Citation7 Only slight differences were observed when HA-hydrogels were implanted in the ear as compared with the kidney. These differences included increased renal engraftment of EPC when HA-hydrogels were implanted in the kidney while ear implants and subsequent EPC release resulted in a slightly better improvement of systemic functions, such as blood pressure.
Table 1. The improvement in various systemic and local parameters during sepsis- and Adriamycin-induced AKI after treatment by adoptive transfer of EPC by either conventional IV delivery or implantation of HA-hydrogels with embedded EPC
Table 2. The summary of systemic and renal effects when EPC are delivered by HA-hydrogel embedding and transplantation as compared with conventional IV injection during AKI
We further examined the effects of EPC delivery by HA-hydrogel to determine if the enhanced therapeutic effect of these bioengineered scaffolds was applicable to other kidney injury models in addition to Adriamycin nephropathy. In a mouse renal ischemia-reperfusion injury (IRI) model of AKI, in which renal blood flow was occluded for 30 min causing microcirculatory and tubular damage, the delivery of EPC by HA-hydrogel resulted in 50% attenuation of the associated rise in serum creatinine 36 h post-IRI, demonstrating the beneficial effects of HA-hydrogel delivery of EPC are not limited to Adriamycin-induced AKI.Citation7 EPC have been shown to mediate re-vascularization and angiogenesis in models of vascular injury.Citation7,Citation10,Citation11,Citation31 Improvement in microvascular competence and function plays a role in the renoprotective effects offered by EPC during AKI. When EPC were delivered into a model of vascular injury (a murine model of hind limb femoral ligation), conventional IV delivery of EPC improved neovascularization by 25% while HA-hydrogel delivery of EPC improved neovascularization and angiogenesis by 45%, further illustrating enhancement of vascular reparative effects mediated by HA-hydrogel delivery.Citation7
The improvement in the therapeutic efficacy of adoptive transfer of EPC by HA-hydrogel delivery prompted us to examine the effects of this phenomenon more elaborately in another model of AKI. Endotoxemia and related sepsis is a growing health problem in our society today and is a significant contributor to AKI. During sepsis, circulating endotoxins cause damage to the vascular endothelium and promote a cataclysmic immune system response that involves substantial release of chemokines and cytokines causing multiple organ damage including AKI.Citation32,Citation33 Since sepsis-induced AKI is a result of microvascular damage and the immune response, the ability of EPC to mediate both vascular repair/regeneration while also modulating the immune response makes these stem/progenitor cells attractive candidates for cell therapy of this form of AKI. A critical effect that occurs during sepsis is that intrinsic EPC niches and reparative stem cell pools are adversely affected by circulating endotoxins including loss of endogenous EPC competence, integrity and viability leading to the inability of these stem cells to mediate vascular and renal repair and regeneration.Citation34-Citation36 While adoptive transfer of EPC is an attractive approach to combat sepsis-induced AKI, conventional IV delivery of these cells is problematic because once EPC are introduced into the circulation, they are predisposed to damage due to their exposure to the circulating endotoxins initially responsible for both systemic and local tissue damage.
Similar to our studies on Adriamycin, HA-hydrogels protected embedded EPC from the endotoxin LPS in in vitro experiments. In in vivo studies, adoptive transfer of EPC to septic mice improved systemic functions within 24 h after injection of LPS.Citation2 Sepsis is typically characterized by hypotension and elevation of circulating hepatic enzymes.Citation2,Citation32 EPC delivery by IV injection significantly improved arterial blood pressure and reduced hepatic release of ALT and AST, however, EPC delivery by HA-hydrogel completely normalized both blood pressure and hepatic enzyme release to healthy baseline levels.Citation2 EPC adoptive transfer also afforded renoprotection, both short- and long-term during sepsis. Impaired renal hemodynamics has been found to be crucially involved in kidney injury during sepsis.Citation37 IV administration of EPC improved renal blood flow, both cortical and medullary, by 40% and 22%, respectively; however, this improvement was markedly potentiated to 75% and 56% when EPC were delivered by HA-hydrogel. In addition to improving renal microcirculation, renal function (as measured by serum creatinine) was improved by 55% upon HA-hydrogel EPC delivery.Citation2 In long-term studies, EPC delivery by HA-hydrogel cut in half the renal interstitial fibrosis that was associated with sepsis-induced AKI and endogenous bone marrow EPC competence was restored.Citation2 While adoptive transfer of EPC in general led to improvement of all sepsis-induced systemic and renal abnormalities, the therapeutic ability of EPC was greatly enhanced (both short- and long-term) when these cells were delivered by HA-hydrogel scaffolding. The duration of a latent period before cells are released from the implanted HA-hydrogel scaffold and become exposed to the circulating endotoxins represents the major difference between the adoptive transfer of cells via HA-hydrogel vs. IV delivery.
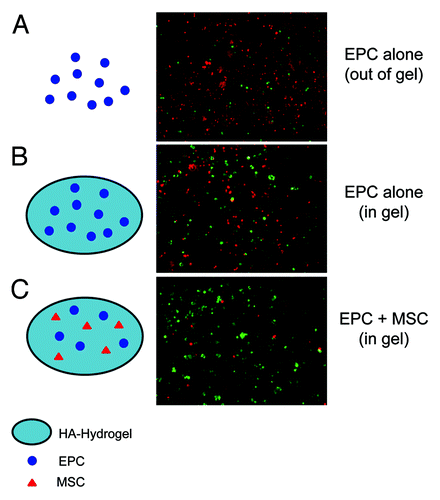
In the latest series of hydrogel experiments by our lab, we have combined delivery of EPC with renal mesenchymal stem cells (MSC). Our lab has previously isolated MSC from the murine kidney.Citation38 These MSC are positive for typical stem cell markers such as Sca-1, CD29, CD44, CD73, negative for CD117 and CD45, and are able to differentiate into multiple cell lineages including chondrocytes, osteocytes and adipocytes.Citation38 Intrinsic stem cell niches, such as in bone marrow and in cardiac tissue, contain multiple progenitor cell types and lineage committed cells which serve as supporting cells for the niche.Citation39-Citation41 Supporting cells help maintain the quality and stability of the niche and play a role in asymmetric stem cell division and renewal of daughter cells.Citation39-Citation41 Introducing renal MSC into HA-hydrogels to support EPC may improve the quality of the bioartificial niche and enhance the regenerative response of the embedded cells. MSC are especially attractive candidates for the supporting cell role because they have been reported to improve stem cell niche quality and stem cell mobilization and homing through release of SDF-1, SCF and LIF.Citation42 MSC also possess immuno-modulatory properties due to their release of anti-inflammatory molecules, such as IL-10, and are capable of inducing neighbor cells to secrete cytokines which may inhibit inflammation and pathological remodeling.Citation42 The therapeutic effects of MSC were recently further demonstrated in a study by Gao et al., in which renal delivery of adipose-derived MSC embedded in a chitosan based hydrogel improved renal function in an IRI model of AKI, an effect that included reduced renal cell apoptosis, improved microvessel density and enhanced tubular cell proliferation.Citation43 The study by Gao et al. supports the use of hydrogels for therapeutic delivery of stem cells and shows the hydrogel scaffold capable supporting delivery of various stem cell lines. To this end, we rationalized that by embedding MSC along with EPC into HA-hydrogels destined for implantation, the therapeutic efficacy of EPC may be enhanced even farther than if EPC were embedded and delivered alone. In in vitro studies, we observed significant improvement in the resistance of EPC to endotoxins (LPS) when MSC were embedded in combination with EPC, as opposed to EPC embedding alone () (our unpublished observations). In in vivo studies on sepsis-induced AKI, delivery of EPC in HA-hydrogels in combination with MSC led to greater improvement in renal microcirculation and renal function, compared with EPC delivered alone. While we are currently investigating the mechanism(s) of beneficial effects that MSC have on EPC, it appears these effects are paracrine in nature and are mediated by the release of protective molecules.
Figure 2.Schematic representation (left) and corresponding images (right) (40x magnification) of EPC treated with 10 ug/ml LPS for 24 h. During LPS treatment, EPC were plated on culture plates (A) (without HA-hydrogel embedding), embedded in HA-hydrogels (B), or embedded in HA-hydrogels along with MSC (C). Embedding EPC in HA-hydrogels improved EPC viability and resistance to endotoxins, an effect that was considerably enhanced when EPC were co-embedded with MSC. To determine cell viability, cells were subject to a LIVE/DEAD assay in which live cells were stained green with calcein and dead cells were stained red with ethidium homodimer.
In our experiments using various AKI models, HA-hydrogel delivery of EPC resulted in enhanced renal engraftment of these cells, as compared with IV delivery. Although improvement in systemic and renal function parallels the difference in engrafting EPC, the relatively low frequency of kidney-lodged cells suggests that this is not the major route responsible for improved renal function and microcirculation. Upon further evaluation, we observed significant reduction of circulating pro-inflammatory chemokines and cytokines when EPC are delivered by HA-hydrogel, an effect that concomitantly accompanied improvement of systemic and renal function. Delivery of EPC by HA-hydrogel greatly attenuated the release of pro-inflammatory molecules such as IL-1α, IL-1β, IL-6, IFNγ and IP-10, to name a few.Citation2 The reduction of the pro-inflammatory response that accompanies sepsis may in large part mediate the improvement in systemic hemodynamics, renal microcirculation and endothelial integrity, factors that all improve renal function. While HA-hydrogel embedded EPC are protected from circulating endotoxins, these cells are still able to secrete anti-inflammatory and pro-angiogenic molecules that can escape the scaffolding into the surrounding tissue and circulation thereby eliciting a pro-reparative effect. This may be particularly true for MSC which are capable of abundant release of anti-inflammatory and pro-angiogenic paracrine molecules such IL-10, IL-8, IP-10, MMP’s and various growth factors including VEGF, bFGF and HGH.Citation42
Conclusions
EPC delivery in bioengineered scaffolds, such HA-hydrogels, offers significant advantages over conventional cell delivery by IV injection. Pronectin-coated HA-hydrogels closely resemble endogenous stem cell niches and thus provide a microenvironment conducive for viability and expansion of embedded cells while also providing protection from circulating cyto- and endo-toxins. HA-hydrogels are readily implantable within tissues and organs, including the kidney. The composition of HA-hydrogels allows them to be easily dissolved on demand permitting mobilization of embedded cells. In experiments using multiple models of AKI, the use of HA-hydrogels for delivery of EPC enhances the therapeutic efficacy of these stem cells by providing substantial systemic and renal protective effects. The enhanced therapeutic performance of HA-hydrogel embedded EPC is the result of multiple factors. Increased cellular α4β1 integrin binding to RGD sequences present in HA-hydrogels activate EPC causing potentiated release of anti-inflammatory and pro-angiogenic molecules, engraftment and transdifferentiation. Despite current advancements, continued development of the adoptive transfer of EPC by HA-hydrogel delivery is needed before its application is clinically realized for treatment of AKI.
| Abbreviations: | ||
| AKI | = | acute kidney injury |
| EPC | = | endothelial progenitor cells |
| βFGF | = | beta fibroblast growth factor |
| HA | = | hyaluronic acid |
| HGH | = | human growth hormone |
| IFNγ | = | interferon gamma |
| IL | = | interleukin |
| IP-10 | = | Interferon gamma-induced protein 10 |
| IRI | = | ischemia-reperfusion injury |
| IV | = | intravenous |
| LIF | = | leukemia inhibitory factor |
| LPS | = | lipopolysaccharide |
| MMP | = | matrix metalloproteinase |
| MSC | = | mesenchymal stem cells |
| PEGDA | = | polyethylene (glycol) diacrylate |
| RGD | = | arginine-glycine-aspartic acid sequence |
| SCF | = | stem cell factor |
| SDF-1 | = | stromal-cell derived factor 1 |
| VEGF | = | vascular endothelial growth factor |
Acknowledgments
Studies from the authors’ laboratories were supported in part by the grants from the AHA 12SDG9080006 (BBR) and NIH DK54602, DK052783, DK45462 and Westchester Artificial Kidney Foundation (MSG).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Baddour JA, Sousounis K, Tsonis PA. Organ repair and regeneration: an overview. Birth Defects Res C Embryo Today 2012; 96:1 - 29; http://dx.doi.org/10.1002/bdrc.21006; PMID: 22457174
- Ghaly T, Rabadi MM, Weber M, Rabadi SM, Bank M, Grom JM, et al. Hydrogel-embedded endothelial progenitor cells evade LPS and mitigate endotoxemia. Am J Physiol Renal Physiol 2011; 301:F802 - 12; http://dx.doi.org/10.1152/ajprenal.00124.2011; PMID: 21775481
- Kale S, Karihaloo A, Clark PR, Kashgarian M, Krause DS, Cantley LG. Bone marrow stem cells contribute to repair of the ischemically injured renal tubule. J Clin Invest 2003; 112:42 - 9; PMID: 12824456
- Park HC, Yasuda K, Ratliff B, Stoessel A, Sharkovska Y, Yamamoto I, et al. Postobstructive regeneration of kidney is derailed when surge in renal stem cells during course of unilateral ureteral obstruction is halted. Am J Physiol Renal Physiol 2010; 298:F357 - 64; http://dx.doi.org/10.1152/ajprenal.00542.2009; PMID: 19906947
- Patschan D, Plotkin M, Goligorsky MS. Therapeutic use of stem and endothelial progenitor cells in acute renal injury: ça ira. Curr Opin Pharmacol 2006; 6:176 - 83; http://dx.doi.org/10.1016/j.coph.2005.10.013; PMID: 16487748
- Pino CJ, Humes HD. Stem cell technology for the treatment of acute and chronic renal failure. Transl Res 2010; 156:161 - 8; http://dx.doi.org/10.1016/j.trsl.2010.07.005; PMID: 20801413
- Ratliff BB, Ghaly T, Brudnicki P, Yasuda K, Rajdev M, Bank M, et al. Endothelial progenitors encapsulated in bioartificial niches are insulated from systemic cytotoxicity and are angiogenesis competent. Am J Physiol Renal Physiol 2010; 299:F178 - 86; http://dx.doi.org/10.1152/ajprenal.00102.2010; PMID: 20410213
- Yasuda K, Park HC, Ratliff B, Addabbo F, Hatzopoulos AK, Chander P, et al. Adriamycin nephropathy: a failure of endothelial progenitor cell-induced repair. Am J Pathol 2010; 176:1685 - 95; http://dx.doi.org/10.2353/ajpath.2010.091071; PMID: 20167859
- Goligorsky MS, Yasuda K, Ratliff B. Dysfunctional endothelial progenitor cells in chronic kidney disease. J Am Soc Nephrol 2010; 21:911 - 9; http://dx.doi.org/10.1681/ASN.2009111119; PMID: 20395371
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997; 275:964 - 7; http://dx.doi.org/10.1126/science.275.5302.964; PMID: 9020076
- Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A 2000; 97:3422 - 7; http://dx.doi.org/10.1073/pnas.97.7.3422; PMID: 10725398
- Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation 2002; 105:1541 - 4; http://dx.doi.org/10.1161/01.CIR.0000013836.85741.17; PMID: 11927518
- Young PP, Vaughan DE, Hatzopoulos AK. Biologic properties of endothelial progenitor cells and their potential for cell therapy. Prog Cardiovasc Dis 2007; 49:421 - 9; http://dx.doi.org/10.1016/j.pcad.2007.02.004; PMID: 17498522
- Caiado F, Dias S. Endothelial progenitor cells and integrins: adhesive needs. Fibrogenesis Tissue Repair 2012; 5:4; http://dx.doi.org/10.1186/1755-1536-5-4; PMID: 22410175
- Franke K, Pompe T, Bornhäuser M, Werner C. Engineered matrix coatings to modulate the adhesion of CD133+ human hematopoietic progenitor cells. Biomaterials 2007; 28:836 - 43; http://dx.doi.org/10.1016/j.biomaterials.2006.09.031; PMID: 17034846
- Li YJ, Chung EH, Rodriguez RT, Firpo MT, Healy KE. Hydrogels as artificial matrices for human embryonic stem cell self-renewal. J Biomed Mater Res A 2006; 79:1 - 5; http://dx.doi.org/10.1002/jbm.a.30732; PMID: 16741988
- Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix--cytoskeleton crosstalk. Nat Rev Mol Cell Biol 2001; 2:793 - 805; http://dx.doi.org/10.1038/35099066; PMID: 11715046
- Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol 2005; 23:47 - 55; http://dx.doi.org/10.1038/nbt1055; PMID: 15637621
- Peattie RA, Pike DB, Yu B, Cai S, Shu XZ, Prestwich GD, et al. Effect of gelatin on heparin regulation of cytokine release from hyaluronan-based hydrogels. Drug Deliv 2008; 15:389 - 97; http://dx.doi.org/10.1080/10717540802035442; PMID: 18686083
- Leach JK. Multifunctional cell-instructive materials for tissue regeneration. Regen Med 2006; 1:447 - 55; http://dx.doi.org/10.2217/17460751.1.4.447; PMID: 17465837
- Burdick JA, Prestwich GD. Hyaluronic acid hydrogels for biomedical applications. Adv Mater 2011; 23:H41 - 56; http://dx.doi.org/10.1002/adma.201003963; PMID: 21394792
- Levenberg S, Burdick JA, Kraehenbuehl T, Langer R. Neurotrophin-induced differentiation of human embryonic stem cells on three-dimensional polymeric scaffolds. Tissue Eng 2005; 11:506 - 12; http://dx.doi.org/10.1089/ten.2005.11.506; PMID: 15869429
- Zhang J, Skardal A, Prestwich GD. Engineered extracellular matrices with cleavable crosslinkers for cell expansion and easy cell recovery. Biomaterials 2008; 29:4521 - 31; http://dx.doi.org/10.1016/j.biomaterials.2008.08.008; PMID: 18768219
- Basu J, Genheimer CW, Rivera EA, Payne R, Mihalko K, Guthrie K, et al. Functional evaluation of primary renal cell/biomaterial Neo-Kidney Augment prototypes for renal tissue engineering. Cell Transplant 2011; In press http://dx.doi.org/10.3727/096368911X566172; PMID: 21439130
- Cai S, Liu Y, Zheng Shu X, Prestwich GD. Injectable glycosaminoglycan hydrogels for controlled release of human basic fibroblast growth factor. Biomaterials 2005; 26:6054 - 67; http://dx.doi.org/10.1016/j.biomaterials.2005.03.012; PMID: 15958243
- Elia R, Fuegy PW, VanDelden A, Firpo MA, Prestwich GD, Peattie RA. Stimulation of in vivo angiogenesis by in situ crosslinked, dual growth factor-loaded, glycosaminoglycan hydrogels. Biomaterials 2010; 31:4630 - 8; http://dx.doi.org/10.1016/j.biomaterials.2010.02.043; PMID: 20227760
- Hosack LW, Firpo MA, Scott JA, Prestwich GD, Peattie RA. Microvascular maturity elicited in tissue treated with cytokine-loaded hyaluronan-based hydrogels. Biomaterials 2008; 29:2336 - 47; http://dx.doi.org/10.1016/j.biomaterials.2008.01.033; PMID: 18313745
- Bailey AS, Jiang S, Afentoulis M, Baumann CI, Schroeder DA, Olson SB, et al. Transplanted adult hematopoietic stems cells differentiate into functional endothelial cells. Blood 2004; 103:13 - 9; http://dx.doi.org/10.1182/blood-2003-05-1684; PMID: 12958072
- Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res 2001; 89:E1 - 7; http://dx.doi.org/10.1161/hh1301.093953; PMID: 11440984
- Bertani T, Poggi A, Pozzoni R, Delaini F, Sacchi G, Thoua Y, et al. Adriamycin-induced nephrotic syndrome in rats: sequence of pathologic events. Lab Invest 1982; 46:16 - 23; PMID: 6172662
- Urbich C, Heeschen C, Aicher A, Dernbach E, Zeiher AM, Dimmeler S. Relevance of monocytic features for neovascularization capacity of circulating endothelial progenitor cells. Circulation 2003; 108:2511 - 6; http://dx.doi.org/10.1161/01.CIR.0000096483.29777.50; PMID: 14581410
- Aird WC. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood 2003; 101:3765 - 77; http://dx.doi.org/10.1182/blood-2002-06-1887; PMID: 12543869
- Sakr Y, Dubois MJ, De Backer D, Creteur J, Vincent JL. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med 2004; 32:1825 - 31; http://dx.doi.org/10.1097/01.CCM.0000138558.16257.3F; PMID: 15343008
- Beck GC, Rafat N, Yard B, Hanusch C. [The role of endothelial progenitor cells in sepsis]. Anaesthesist 2007; 56:423 - 8; http://dx.doi.org/10.1007/s00101-007-1183-z; PMID: 17443298
- Mayr FB, Spiel AO, Leitner JM, Firbas C, Sieghart W, Jilma B. Effects of low dose endotoxemia on endothelial progenitor cells in humans. Atherosclerosis 2007; 195:e202 - 6; http://dx.doi.org/10.1016/j.atherosclerosis.2007.04.003; PMID: 17490672
- Yamada M, Kubo H, Kobayashi S, Ishizawa K, Numasaki M, Ueda S, et al. Bone marrow-derived progenitor cells are important for lung repair after lipopolysaccharide-induced lung injury. J Immunol 2004; 172:1266 - 72; PMID: 14707105
- Doi K, Leelahavanichkul A, Yuen PS, Star RA. Animal models of sepsis and sepsis-induced kidney injury. J Clin Invest 2009; 119:2868 - 78; http://dx.doi.org/10.1172/JCI39421; PMID: 19805915
- Plotkin MD, Goligorsky MS. Mesenchymal cells from adult kidney support angiogenesis and differentiate into multiple interstitial cell types including erythropoietin-producing fibroblasts. Am J Physiol Renal Physiol 2006; 291:F902 - 12; http://dx.doi.org/10.1152/ajprenal.00396.2005; PMID: 16622175
- Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 2005; 121:1109 - 21; http://dx.doi.org/10.1016/j.cell.2005.05.026; PMID: 15989959
- Moore KA, Lemischka IR. Stem cells and their niches. Science 2006; 311:1880 - 5; http://dx.doi.org/10.1126/science.1110542; PMID: 16574858
- Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 2003; 425:836 - 41; http://dx.doi.org/10.1038/nature02041; PMID: 14574412
- Ranganath SH, Levy O, Inamdar MS, Karp JM. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell 2012; 10:244 - 58; http://dx.doi.org/10.1016/j.stem.2012.02.005; PMID: 22385653
- Gao J, Liu R, Wu J, Liu Z, Li J, Zhou J, et al. The use of chitosan based hydrogel for enhancing the therapeutic benefits of adipose-derived MSCs for acute kidney injury. Biomaterials 2012; 33:3673 - 81; http://dx.doi.org/10.1016/j.biomaterials.2012.01.061; PMID: 22361096