Abstract
Tor (Target of Rapamycin) pathway underlies a major signaling mechanism for controlling cell growth and proliferation1. Rheb (Ras homolog enriched in brain) is a small GTPase in the Tor pathway2-4. Similar to other small GTPases, Rheb cycles between a GTP-bound active state and a GDP-bound inactive state. TSC2 (Tuberous sclerosis complex 2), a gene mutated in an autosomal dominant disease Tuberous sclerosis, was shown to be the Rheb-GAP (GTPase activating protein)5, 6. However, a guanine nucleotide exchange factor (GEF) for Rheb had been missing. Human TCTP (Translationally controlled tumor protein) has been implicated in cancer, but its function in vivo has not been clearly elucidated. Recently we reported a molecular genetic characterization of TCTP function in Drosophila7. Drosophila TCTP (dTCTP) displays GEF activity to Rheb and is essential for Rheb activation in organ growth. Thus, our study provides a tight linkage of dTCTP to the Rheb-TOR pathway. In this addendum, we will briefly overview our findings and discuss our perspectives for future research on TCTP.
TCTP is a highly conserved protein identified about 20 years ago as a translationally controlled protein P21 (or P23) enriched in tumor cell lines.Citation8 Recently, this protein has drawn special interests because of its potential roles in tumorigenesis. TCTP is not only upregulated in a number of tumor cell lines but also downregulated during tumor reversion.Citation9,Citation10 TCTP has also been implicated in a variety of intracellular and extracellular functions, including microtubule stabilization, cell cycle, apoptosis, and cytokine release.Citation11–Citation16 However, these functions of TCTP have been inferred mainly from biochemical interactions and cell culture studies.
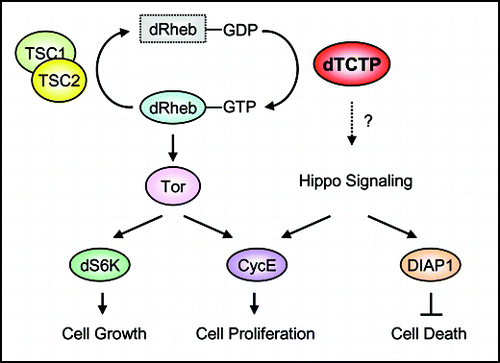
To address the function of TCTP in vivo, we took a loss-of-function approach using Drosophila as a genetic model. Reduction of dTCTP by tissue-specific RNA interference (RNAi) or loss-of-function mutations resulted in smaller organs with reduction in both cell size and cell number, a phenotype often seen in mutations in Tor or insulin pathway. Our epistatic analysis suggested that dTCTP acts either downstream or in parallel to insulin receptor, TSC1, and dRheb, but upstream of dS6K. Despite the conserved sequence of TCTP proteins in a wide-range of species, TCTP has little similarity to the sequences of other protein families. However, the three-dimensional structure of fission yeast TCTP ortholog reveals similarities with a family of proteins that bind to the nucleotide-free form of Rab GTPases,Citation17 providing a clue for its potential biochemical function. Consistent with our genetic evidence, our biochemical assays showed that dTCTP could facilitate the GDP/GTP exchange on dRheb both in vitro and in vivo. Our data led us to propose a model () in which dTCTP regulates the Tor signaling pathway by directly interacting with dRheb GTPase as a GEF.
This study not only provides new insights into the mechanism of dTCTP function in regulation of dRheb activity but also reveals a complexity of dTCTP function in growth regulation. Firstly, dTCTP null mutant displays more severe phenotypes than loss of function mutations in the insulin or Tor pathways. For example, while dTCTP null mutant clones are eliminated during development, significant portions of dRheb and Tor null mutant clones can survive to form adult tissues. This argues against the model that dTCTP functions only in regulating the Rheb-Tor pathways. Although different maternal contribution of each gene and variations in genetic background might partially account for these differences, it is equally possible that dTCTP functions as a GEF for more than one GTPase targets. Conversely, other GEFs might exist for Rheb, as one small GTPase can be regulated by more than one GEFs.Citation18,Citation19 Different GEFs might be required for dRheb-TOR function in other developmental events or growth-independent processes like axon guidance during neural development.Citation20
Secondly, in contrast to the implicated role of TCTP in cancer,Citation9 ubiquitous or tissue-specific overexpression of dTCTP was insufficient to cause overgrowth phenotypes in Drosophila. A plausible explanation is that the amount of dTCTP is in excess for dRheb activation in contrast to limited concentrations of TSC2 GAP in normal cells.Citation5 Since overexpression of dTCTP alone does not result in tumorous overgrowth, it is unlikely that TCTP functions as an oncogene directly under normal condition. However, increased amounts of TCTP in tumor tissues may provide better potency for cells to undergo uncontrolled proliferation and massive overgrowth. In this regard, it will be intriguing to learn if dTCTP can act corporately with other oncogenes. Since the amino acid sequence identity of dTCTP and human TCTP is only 48%, it is also possible that a non-conserved region(s) of human TCTP may be required to induce tumors in human cells. Soon after the publication of our work, knockout of the mouse TCTP was reported. Remarkably, loss of TCTP in mice results in early lethality with smaller sizes of embryos.Citation21 Developmental defects seen in mutant mice may be analogous to the phenotypes of dTCTP mutants in Drosophila, supporting the conserved function of TCTP in growth regulation. However, it is yet to be determined whether there is a common molecular basis for the developmental defects in both systems and whether overexpression of mouse TCTP in transgenic mice can induce tumors.
Lastly, in addition to Tor and insulin signaling pathways, a third pathway consisting of Hippo-Warts protein kinase cascade controls organ size by affecting mainly the cell number.Citation22,Citation23 The Hippo pathway regulates both cell proliferation and cell death by promoting cyclin E expression and downregulating Drosophila Inhibitor of Apoptosis 1 (DIAP1). It is worthy of note that dTCTP also regulates the cell number by affecting cell proliferation and apoptosis. It remains to be determined whether dTCTP and Hippo signaling pathways crosstalk or are independent of each other.
Studies on TCTP suggest that its function is much more complex than what is known and its interactions with a multitude of proteins might underlie this complexity. Biochemical studies have identified several proteins interacting with TCTP. Given the feasibility of Drosophila genetics, the physiological relevance of these protein interactions can now be addressed in the context of normal development. It would also be powerful to take the advantage of Drosophila genetic screens for the identification of novel genes interacting with dTCTP. A more comprehensive understanding of TCTP functions and mechanistic explanations of its intriguing expression profiles in cancers can be expected in years to come.
Abbreviations
| dTCTP | = | Drosophila TCTP |
| Rheb | = | Ras homolog enriched in brain |
| GAP | = | GTPase activating protein |
| Tor | = | target of rapamycin |
| TSC2 | = | tuberous sclerosis complex 2 |
Figures and Tables
Figure 1 A model for dTCTP function in growth control. dRheb GTPase stimulates Tor signaling, which in turn activates dS6K and CycE to regulate cell growth and proliferation, respectively. dRheb GTPase is inactivated by the GAP function of TSC1/2 complex. In contrast, dTCTP activates dRheb GTPases by promoting GDP-GTP exchange. dTCTP might have additional functions independent of the Tor pathway, such as inhibiting cell death. It remains to be determined if dTCTP regulates cell proliferation and cell survival in part through the Hippo signaling.
Addendum to:
References
- Edgar BA. How flies get their size: Genetics meets physiology. Nat Rev Genet 2006; 7:907 - 916
- Stocker H, Radimerski T, Schindelholz B, Wittwer F, Belawat P, Daram P, Breuer S, Thomas G, Hafen E. Rheb is an essential regulator of S6K in controlling cell growth in Drosophila. Nat Cell Biol 2003; 5:559 - 565
- Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev 2003; 17:1829 - 1834
- Saucedo LJ, Gao X, Chiarelli DA, Li L, Pan D, Edgar BA. Rheb promotes cell growth as a component of the insulin/TOR signalling network. Nat Cell Biol 2003; 5:566 - 571
- Zhang Y, Gao X, Saucedo LJ, Ru B, Edgar BA, Pan D. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat Cell Biol 2003; 5:578 - 581
- Li Y, Inoki K, Guan KL. Biochemical and functional characterizations of small GTPase Rheb and TSC2 GAP activity. Mol Cell Biol 2004; 24:7965 - 7975
- Hsu YC, Chern JJ, Cai Y, Liu M, Choi KW. Drosophila TCTP is essential for growth and proliferation through regulation of dRheb GTPase. Nature 2007; 445:785 - 788
- Gross B, Gaestel M, Bohm H, Bielka H. cDNA sequence coding for a translationally controlled human tumor protein. Nucleic Acids Res 1989; 17:8367
- Tuynder M, Susini L, Prieur S, Besse S, Fiucci G, Amson R, Telerman A. Biological models and genes of tumor reversion: Cellular reprogramming through tpt1/TCTP and SIAH-1. Proc Natl Acad Sci USA 2002; 99:14976 - 14981
- Arcuri F, Papa S, Carducci A, Romagnoli R, Liberatori S, Riparbelli MG, Sanchez JC, Tosi P, delVecchio MT. Translationally controlled tumor protein (TCTP) in the human prostate and prostate cancer cells: Expression, distribution, and calcium binding activity. Prostate 2004; 60:130 - 140
- Liu H, Peng HW, Cheng YS, Yuan HS, Yang-Yen HF. Stabilization and enhancement of the antiapoptotic activity of Mcl-1 by TCTP. Mol Cell Biol 2005; 25:3117 - 3126
- Yarm FR. Plk phosphorylation regulates the microtubule-stabilizing protein TCTP. Mol Cell Biol 2002; 22:6209 - 6221
- MacDonald SM, Bhisutthibhan J, Shapiro TA, Rogerson SJ, Taylor TE, Tembo M, Langdon JM, Meshnick SR. Immune mimicry in malaria: Plasmodium falciparum secretes a functional histamine-releasing factor homolog in vitro and in vivo. Proc Natl Acad Sci USA 2001; 98:10829 - 10832
- Yoon T, Kim M, Lee K. Inhibition of Na,K-ATPase-suppressive activity of translationally controlled tumor protein by sorting nexin 6. FEBS Lett 2006; 580:3558 - 3564
- Jung J, Kim M, Kim MJ, Kim J, Moon J, Lim JS, Kim M, Lee K. Translationally controlled tumor protein interacts with the third cytoplasmic domain of Na,K-ATPase alpha subunit and inhibits the pump activity in HeLa cells. J Biol Chem 2004; 279:49868 - 49875
- Cans C, Passer BJ, Shalak V, Nancy-Portebois V, Crible V, Amzallag N, Allanic D, Tufino R, Argentini M, Moras D, Fiucci G, Goud B, Mirande M, Amson R, Telerman A. Translationally controlled tumor protein acts as a guanine nucleotide dissociation inhibitor on the translation elongation factor eEF1A. Proc Natl Acad Sci USA 2003; 100:13892 - 13897
- Thaw P, Baxter NJ, Hounslow AM, Price C, Waltho JP, Craven CJ. Structure of TCTP reveals unexpected relationship with guanine nucleotide-free chaperones. Nat Struct Biol 2001; 8:701 - 704
- Roose JP, Mollenauer M, Ho M, Kurosaki T, Weiss A. Unusual interplay of two types of Ras activators, RasGRP and SOS, establishes sensitive and robust Ras activation in lymphocytes. Mol Cell Biol 2007; 27:2732 - 2745
- Rossman KL, Der CJ, Sondek J. GEF means go: Turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol 2005; 6:167 - 180
- Knox S, Ge H, Dimitroff BD, Ren Y, Howe KA, Arsham AM, Easterday MC, Neufeld TP, O'Connor MB, Selleck SB. Mechanisms of TSC-mediated control of synapse assembly and axon guidance. PLoS ONE 2007; 2:e375
- Ho Chen S, Wu PS, Chou CH, Yan YT, Liu H, Weng SY, Yang-Yen HF. A knockout mouse approach reveals that TCTP functions as an essential factor for cell proliferation and survival in a tissue- or cell type-specific manner. Mol Biol Cell 2007; 18:2525 - 2532
- Hariharan IK. Growth regulation: A beginning for the hippo pathway. Curr Biol 2006; 16R1037 - 16R1039
- Pan D. Hippo signaling in organ size control. Genes Dev 2007; 21:886 - 897